Back
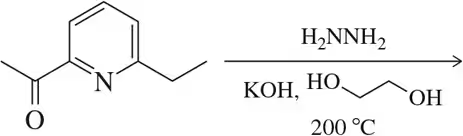
BackProblem 51a
Show the product expected by the Wolff–Kishner reduction of the following aldehydes/ketones.
(a)
Problem 54
During an oxidation reaction, there must also be a reduction. What is reduced in the Pinnick oxidation?
Problem 56a
The following molecules were named incorrectly according to IUPAC nomenclature. Give the correct name of these compounds.
(a) 3-oxo-5-methylhexan-2-ol
Problem 56b
The following molecules were named incorrectly according to IUPAC nomenclature. Give the correct name of these compounds.
b. (R)-6-bromo-7-oxoheptan-2-one
Problem 56d
The following molecules were named incorrectly according to IUPAC nomenclature. Give the correct name of these compounds.
d. (E)-2-methylhex-2-en-6-al
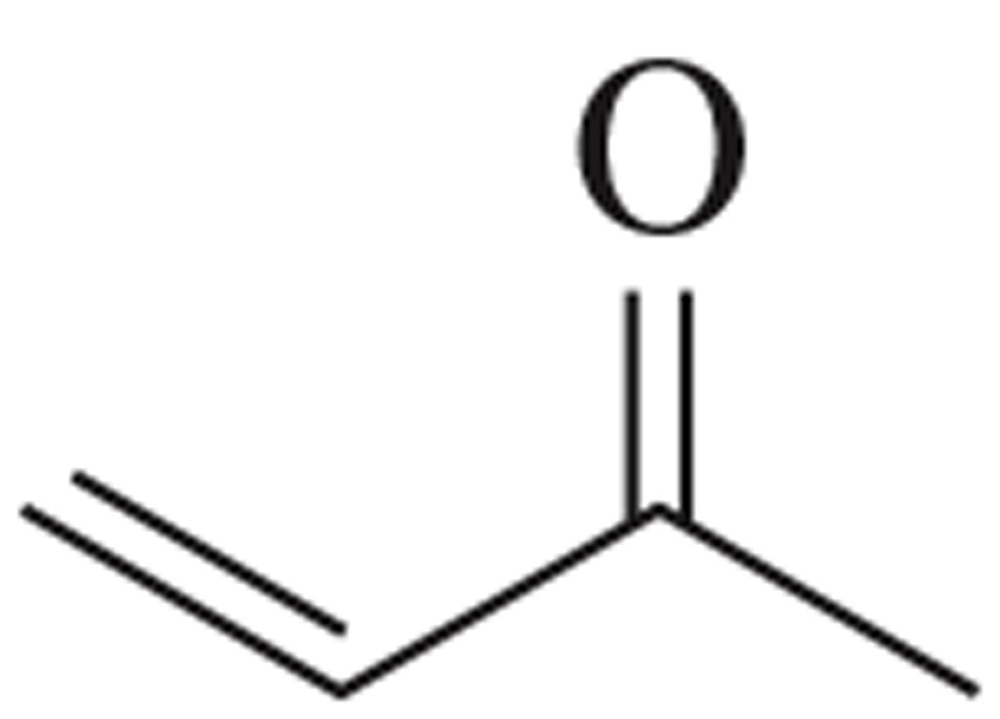
Problem 60
Draw the molecular orbital picture of but-3-en-2-one.
Problem 62
Starting with formaldehyde and the alkyl halides shown as the only sources of carbon, provide a synthesis of the following alcohol.
Problem 64
In lieu of quenching the product of Grignard addition to a carbonyl with acid, alkyl halides can be added directly to generate ether products. Predict the product and show the mechanism of this process.
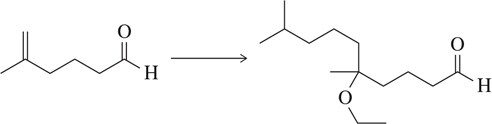
Problem 66a
Suggest a synthetic scheme, involving a protecting group, to generate the molecule shown starting with the molecule at the left.
(a)
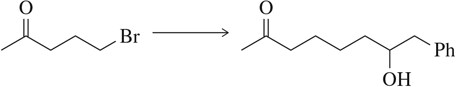
Problem 66b
Suggest a synthetic scheme, involving a protecting group, to generate the molecule shown starting with the molecule at the left.
(b)
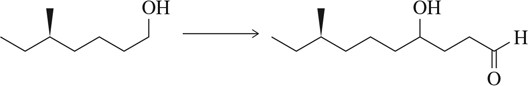
Problem 66c
Suggest a synthetic scheme, involving a protecting group, to generate the molecule shown starting with the molecule at the left.
(c)
Problem 66d
Suggest a synthetic scheme, involving a protecting group, to generate the molecule shown starting with the molecule at the left.
(d)
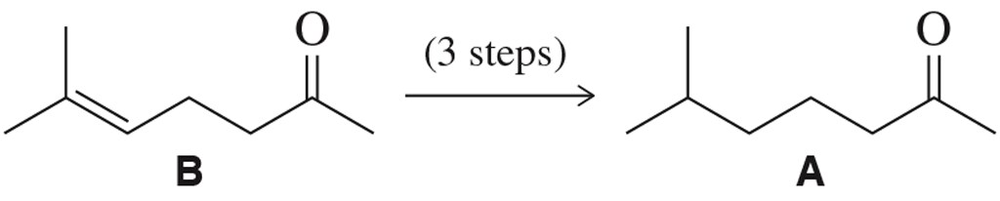
Problem 67
As discussed in Section 17.9.1, alkenes can be hydrogenated selectively in the presence of ketones. Suppose that was not the case, and suggest how you might use a protecting group strategy to generate A from B.
Problem 70
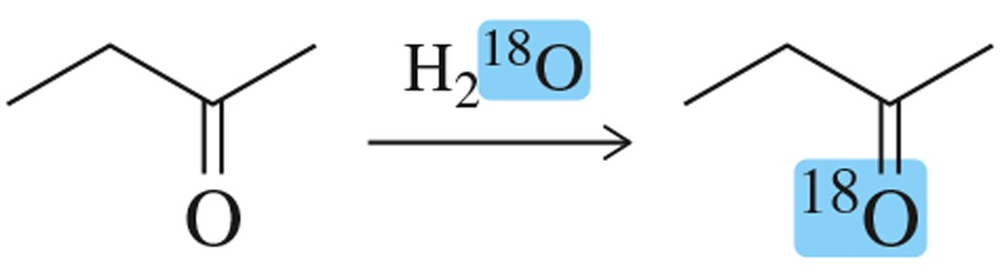
When a ketone is dissolved in 18O-labeled water, the 18O label is incorporated into the ketone. Suggest a mechanism that explains this observation.
Problem 79
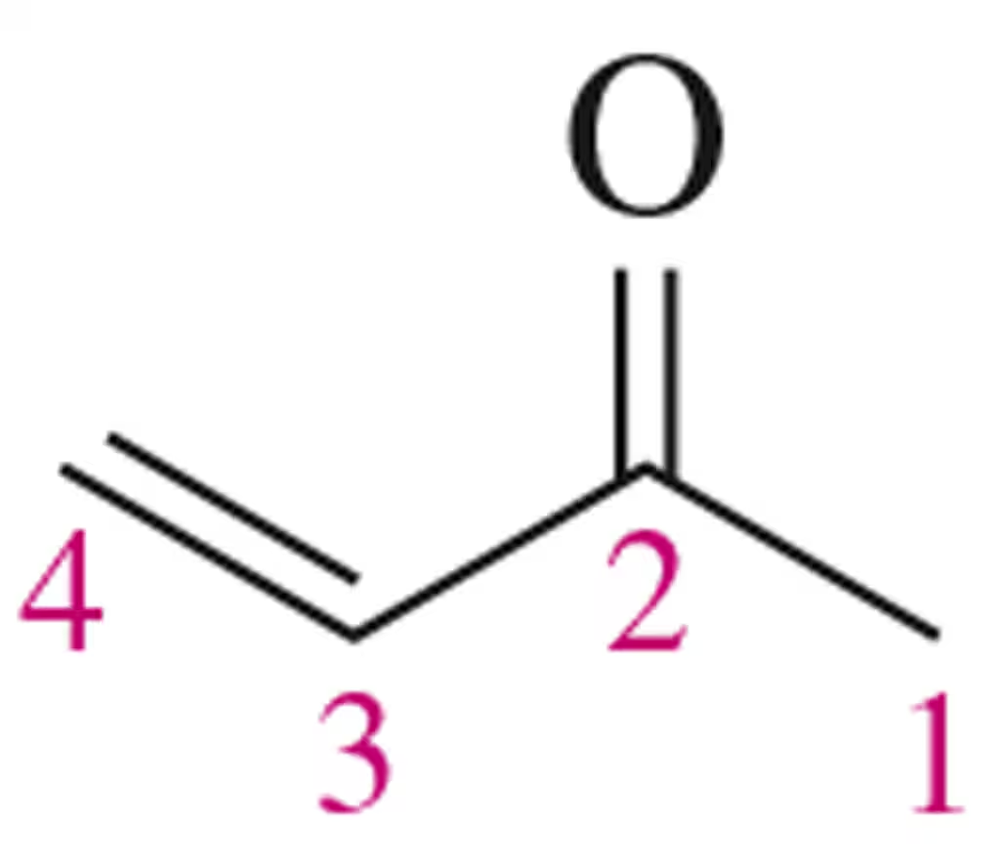
We show in Chapter 20 that α , β-unsaturated ketones are electrophilic at C4. Rationalize this observation.