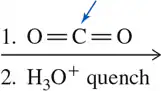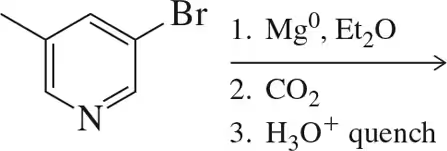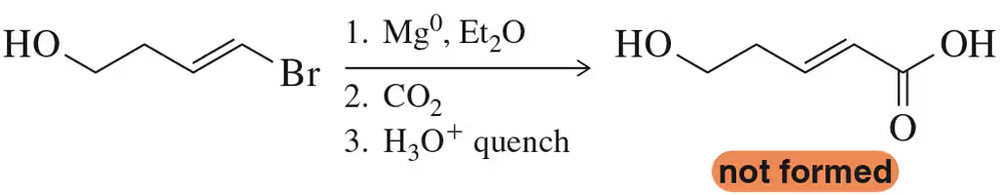Back
BackProblem 3a
Would you expect ∆S to be greater than, less than, or equal to zero in the following reactions?
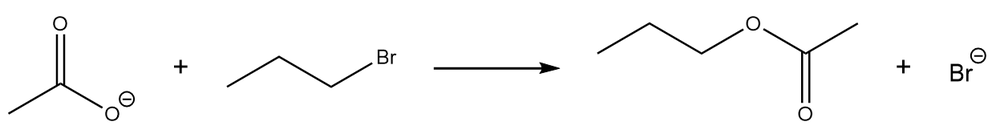
a.
Problem 3b
Would you expect ∆S to be greater than, less than, or equal to zero in the following reactions?
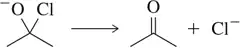
(b)
Problem 4
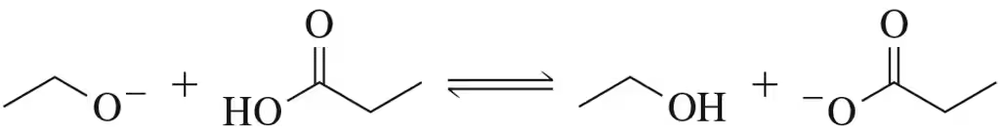
Calculate Keq for the acid–base reaction shown. Which side is favored and why?
Problem 5a
Calculate the oxidation numbers for the indicated atoms.
a.
Problem 5b
Calculate the oxidation numbers for the indicated atoms.
b.
Problem 5c
Calculate the oxidation numbers for the indicated atoms.
(c)
Problem 5d
Calculate the oxidation numbers for the indicated atoms.
(d)
Problem 5e
Calculate the oxidation numbers for the indicated atoms.
(e)
Problem 16a
Predict the product of the following reactions.
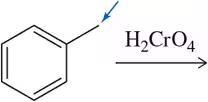
(a)
Problem 20a
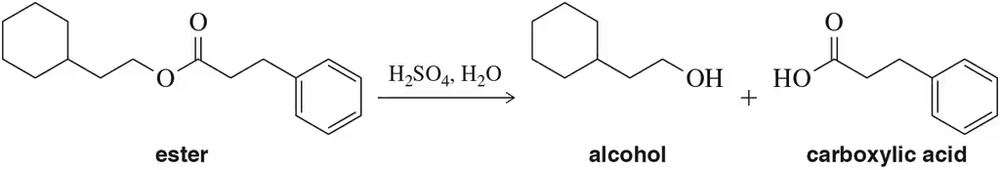
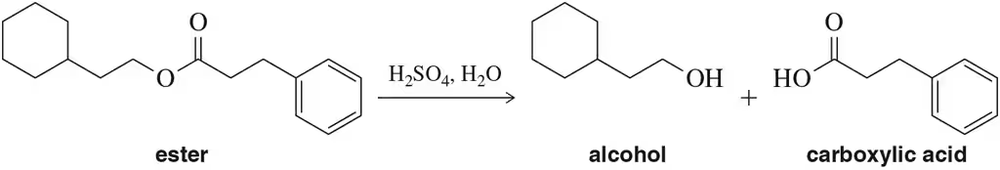
The acid-catalyzed hydrolysis of an ester results in the formation of an equal amount of carboxylic acid and alcohol.
(a) Design a flow chart for separating the two products.
Problem 20b
The acid-catalyzed hydrolysis of an ester results in the formation of an equal amount of carboxylic acid and alcohol.
(b) Once separated, how could you distinguish between the carboxylic acid and alcohol using IR spectroscopy?
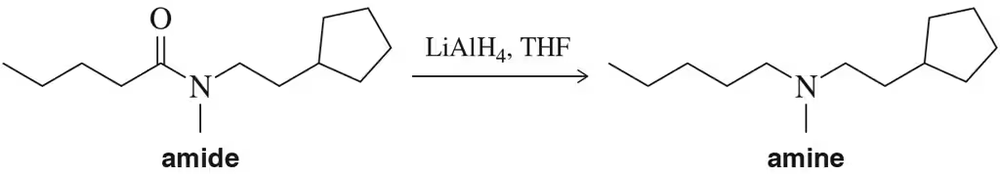
Problem 21
At the end of a reduction, there was found to be a small amount of unreacted amide along with the amine product dissolved in diethyl ether. How might you remove the amine from the ether?
Problem 29
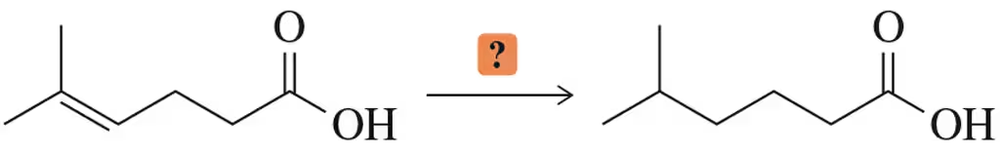
How would you reduce the alkene in the presence of a carboxylic acid?
Problem 36
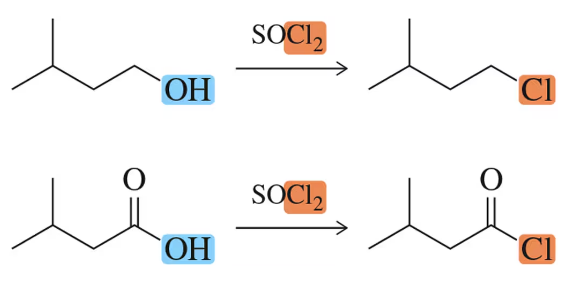
The absence of what band in the IR spectrum of the product in Figure 18.59 would be consistent with full conversion of the carboxylic acid to an acid chloride?
Problem 49b
The following carboxylic acids were named incorrectly. Provide the correct name.
(b) 6-bromocyclohexane carboxylic acid
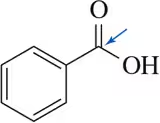
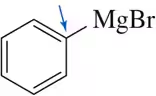
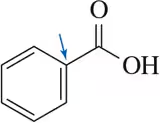
Problem 59
When a chemist attempted the following reaction sequence, the desired product was not formed.
(a) Why?
(b) Suggest a solution to the problem. [Think about chemistry from the end of Chapter 13.]
Problem 68
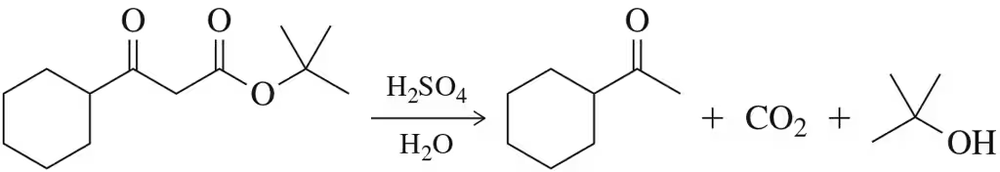
In Chapter 19, we will learn about the hydrolysis of t-butyl esters. In the reaction below, the hydrolysis is coupled to the decarboxylation reaction learned in this chapter. Suggest a mechanism for this reaction. [Hint: The formation of t-butanol proceeds by an SN1 reaction.]
Problem 69
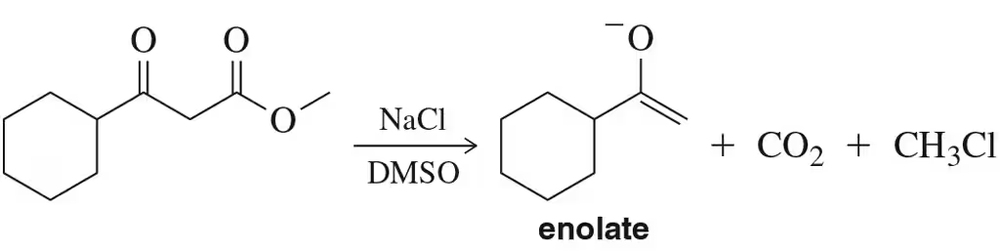
While acidic conditions were used in Assessment 18.68, decarboxylation of esters can also be conducted under basic conditions to give, at least temporarily, the enolate product shown. [We’ll learn more about the chemistry of enolates in Chapter 20.] Suggest a mechanism of this reaction. [Hint: The formation of chloromethane proceeds by an SN2 reaction.]