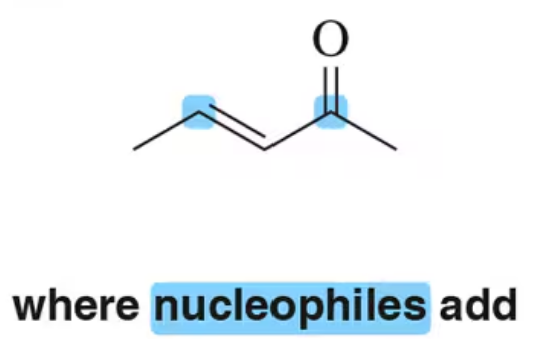
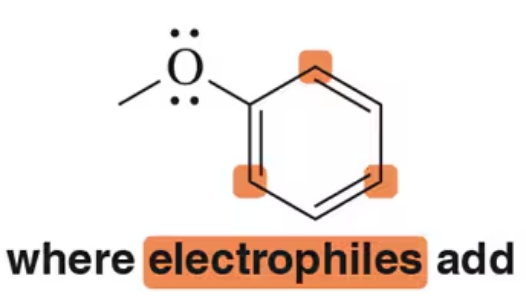
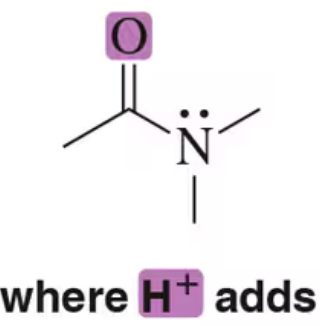
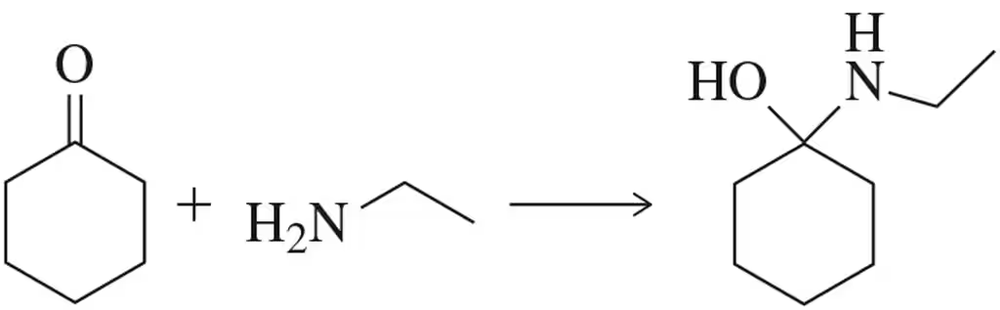
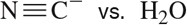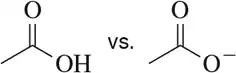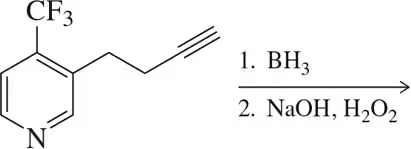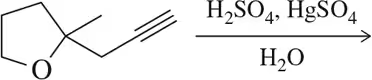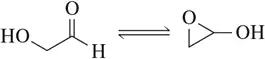Back
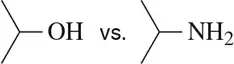
BackProblem 1b
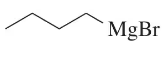
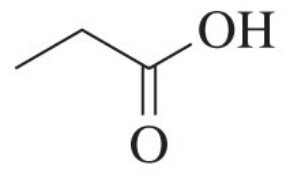
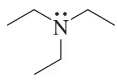
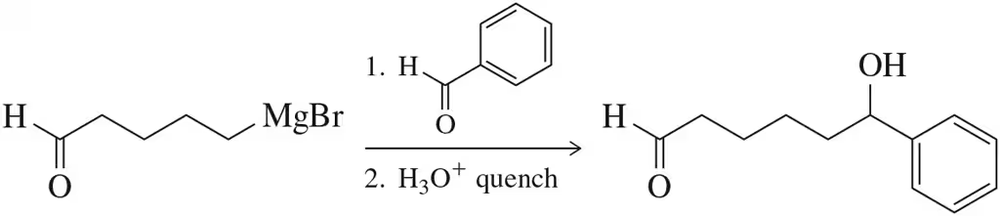
In each case, circle the stronger nucleophile.
(b)
Problem 1c
In each case, circle the stronger nucleophile.
(c)
Problem 1d
In each case, circle the stronger nucleophile.
(d)
Problem 2a
Identify the following reactions as oxidation or reduction (based on what happens to the organic molecule).
(a)
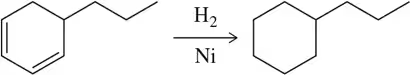
Problem 2b
Identify the following reactions as oxidation or reduction (based on what happens to the organic molecule).
(b)
Problem 2c
Identify the following reactions as oxidation or reduction (based on what happens to the organic molecule).
(c)
Problem 4a
Based on the analysis you used in Assessment 17.3, which carbonyl would you expect to react most quickly with a nucleophile?
(a)
Problem 4c
Based on the analysis you used in Assessment 17.3, which carbonyl would you expect to react most quickly with a nucleophile?
(c)
Problem 7d
Give a structure that corresponds to the name provided.
d. E-4-oxopent-2-enal
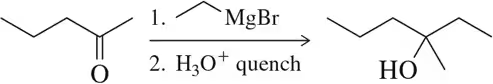
Problem 8a
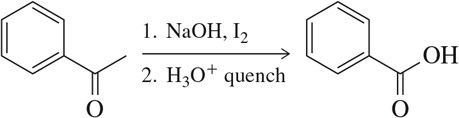
Predict the product of the following aldehyde/ketone syntheses.
(a)
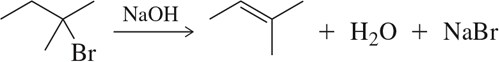
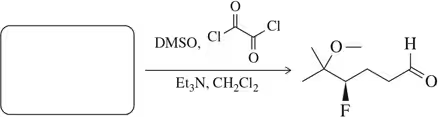
Problem 8c
Predict the product of the following aldehyde/ketone syntheses.
(c)
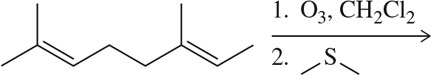
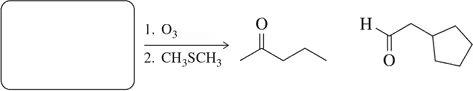
Problem 8d
Predict the product of the following aldehyde/ketone syntheses.
(d)
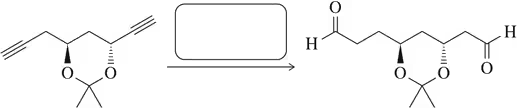
Problem 9a
Predict the reagents or reactant(s) necessary to complete the following syntheses.
(a)
Problem 9b
Predict the reagents or reactant(s) necessary to complete the following syntheses.
(b)
Problem 9c
Predict the reagents or reactant(s) necessary to complete the following syntheses.
(c)
Problem 10
Another way the preceding might be presented is to say that the minor resonance structure(s) reveal the reactivity of a molecule. Show how the minor resonance structure(s) can help us understand that the following reactions occur with the regioselectivity shown.
(a)
(b)
(c)
Problem 11
Identify the bonds broken and formed in the following addition reaction.
(a) Would you expect this reaction to be favored based on entropy?
(b) Based on enthalpy [qualitatively]?
(c) Overall?
Problem 12a
Classify the following nucleophiles as strong, weak, or intermediate. Would you expect each to add to a carbonyl directly or wait for a carbocation to form?
(a)
Problem 12b
Classify the following nucleophiles as strong, weak, or intermediate. Would you expect each to add to a carbonyl directly or wait for a carbocation to form?
b)
Problem 12d
Classify the following nucleophiles as strong, weak, or intermediate. Would you expect each to add to a carbonyl directly or wait for a carbocation to form?
(d)
Problem 17a
A student, when solving the following 'predict-the-product' question, made a common mistake by writing the answer shown here. Explain why this reaction would not work as written.
Problem 20a
Suggest an acetylide ion and a carbonyl that might be used to make the following products.
(a) oct-4-yn-3-ol
Problem 20b
Suggest an acetylide ion and a carbonyl that might be used to make the following products.
(b) 2,6-dimethylhept-3-yn-2-ol
Problem 20c
Suggest an acetylide ion and a carbonyl that might be used to make the following products.
(c) 5-phenylhex-2-yn-1-ol
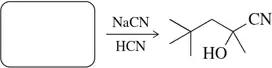
Problem 22a
Suggest a carbonyl to react with NaCN/HCN to produce the following cyanohydrins.
(a)
Problem 23a
For each of the following carbonyl addition reactions, would you expect a racemic mixture or a mixture enriched in one stereoisomer? In each case, draw both possible stereoisomeric products.
(a)
Problem 26a
Suggest the appropriate carbonyl and Wittig reagent to make the following alkenes.
a. (E)-7-methylnon-4-en-3-one
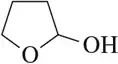
Problem 31c
Identify the hemiacetal functional group in each of the following molecules. These molecules may not be stable enough to be the favorable product in an equilibrium reaction.
(c)
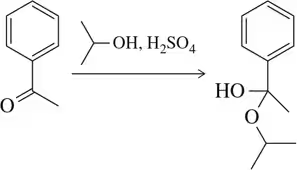
Problem 32a
Provide a mechanism for the formation of the hemiacetals shown. [Only (c) is favored as written.]
(a)
Problem 33a
Which of the following cyclic hemiacetals would you expect to have the highest Keq for their formation? Explain your answer.
(a)