Back
Back Mullins 1st Edition
Mullins 1st Edition Ch. 14 - Structural Identification I: Infrared Spectroscopy and Mass Spectrometry
Ch. 14 - Structural Identification I: Infrared Spectroscopy and Mass SpectrometryProblem 43a
Identify the peaks in the mass spectrum of octan-4-one that correspond to (a) α-cleavage.
<IMAGE>
Problem 43b
Identify the peaks in the mass spectrum of octan-4-one that correspond to (b) the McLafferty rearrangement.
<IMAGE>
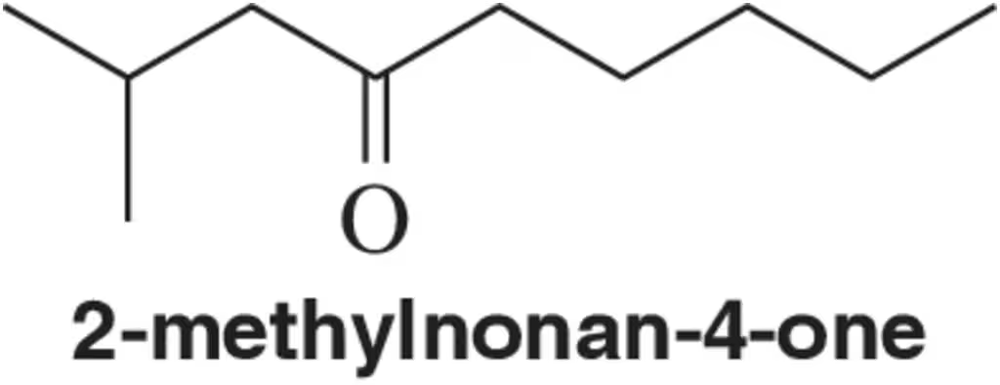
Problem 44
2-Methylnonan-4-one can undergo two different McLafferty rearrangements. Draw the products of each of them.
Problem 45
Assign the structure based on the mass spectrum and IR spectrum shown.
<IMAGE>
Problem 46e
Based on Hooke's law, choose the bond in each pair that you expect to vibrate at a higher wavenumber.
(e) C=N vs C≡N
Problem 46f
Based on Hooke's law, choose the bond in each pair that you expect to vibrate at a higher wavenumber.
(f) C―S vs C=O
Problem 47b
Choose the bond in each pair that you expect to have the more intense stretching band
(b) C=O vs. C=N
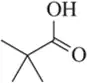
Problem 48a
Identify the important bands you would expect to find in an IR spectrum for the following molecules.
(a)
Problem 48c
Identify the important bands you would expect to find in an IR spectrum for the following molecules.
(c)
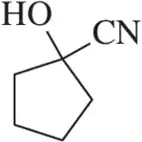
Problem 49b
What functional groups might be present in the IR spectra for the molecules with the given molecular formulas. [Be sure to use the molecular formula in your analysis.]
(b) C₃H₄O₄
<IMAGE>
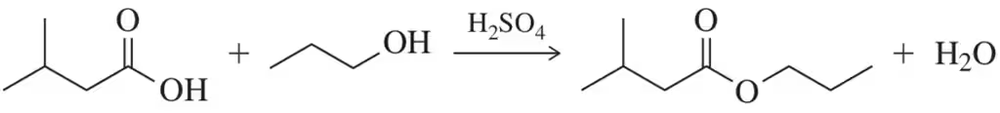
Problem 51
A carboxylic acid can be converted to an ester using the conditions shown. Explain how a comparison of an IR spectrum of the reactant(s) and product can be used to determine whether the reaction was successful or not.
Problem 52
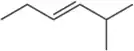
Hoping to make the following diene, a chemist treated the diol shown with acid. Based on the IR spectrum, was the reaction successful? If not, what compound was made instead?
<IMAGE>
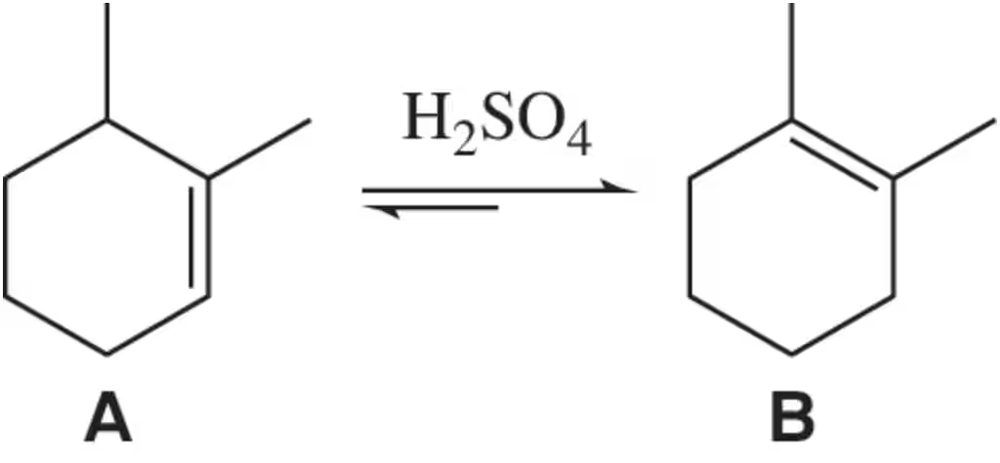
Problem 54
Under acidic conditions, alkene A can be isomerized to the more stable alkene B. How could IR spectroscopy be used to distinguish between A and B? [There are a few correct answers.]
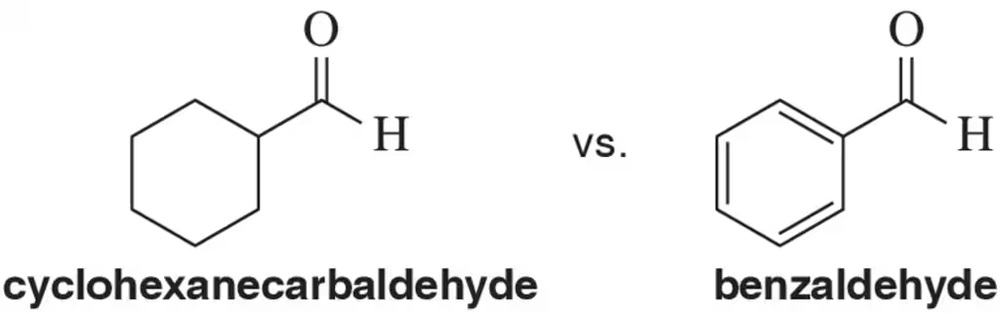
Problem 55
Would you expect the stretching band of the carbonyl to appear at a higher frequency for cyclohexanecarbaldehyde or benzaldehyde? Explain.
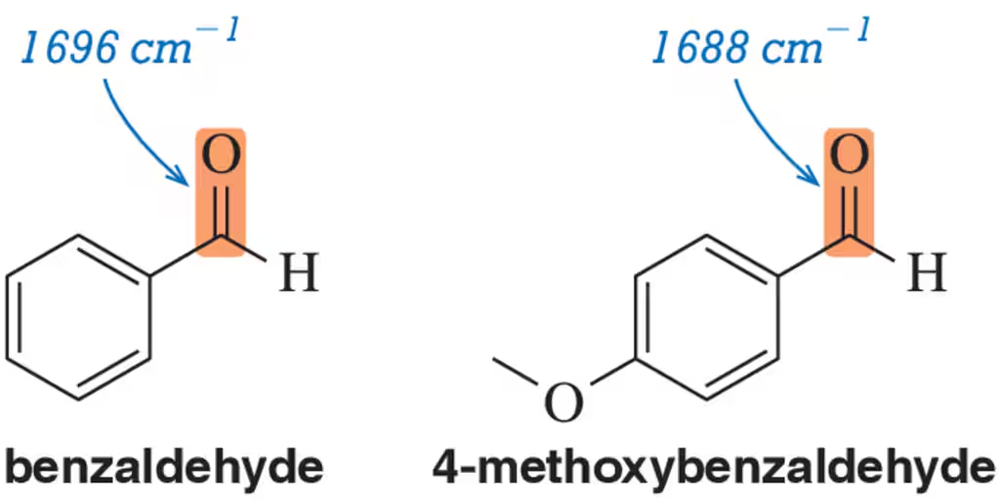
Problem 56
Justify the carbonyl stretching frequencies indicated for benzaldehyde and 4-methoxybenzaldehyde.
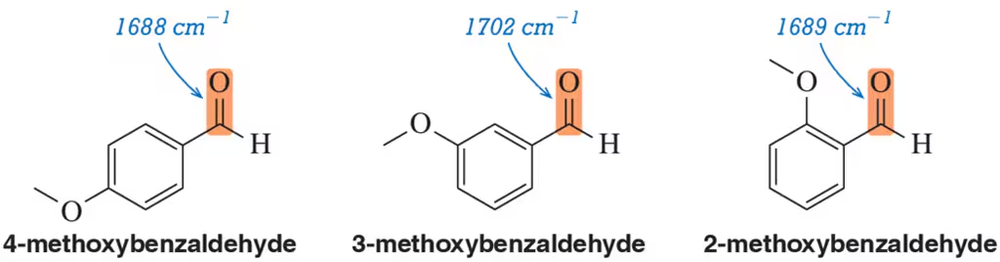
Problem 57
Justify the carbonyl stretching frequencies for a series of methoxybenzaldehydes. Specifically, why are the 2- and 4-methoxy derivatives similar to each other but different from the 3-methoxy derivative?
Problem 58
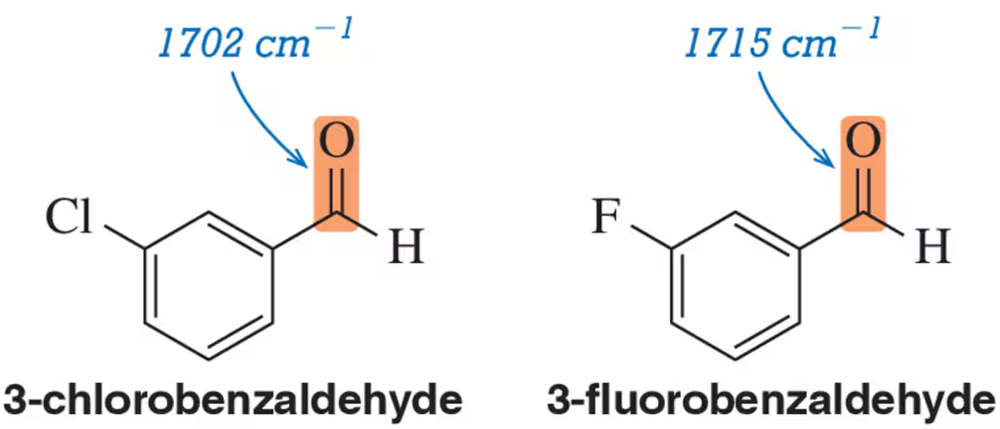
Justify the carbonyl stretching frequencies indicated for 3-chlorobenzaldehyde and 3-fluorobenzaldehyde.
Problem 59
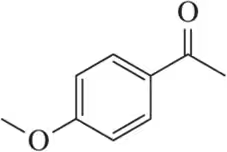
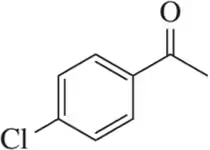
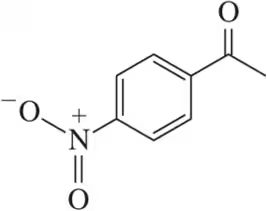
Rank the following acetophenone derivatives on the basis of the carbonyl stretching frequency (1 = highest ; 4 = lowest ).
(a)
(b)
(c)
(d)
Problem 60a
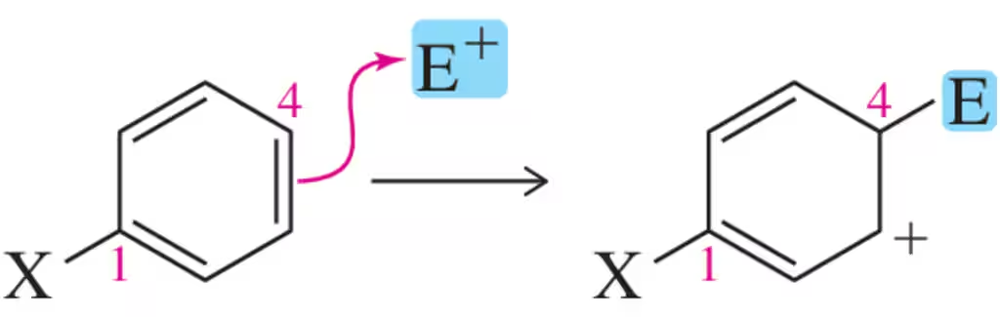
The first step of a reaction called electrophilic aromatic substitution is as follows:
If this step is rate-determining for the overall reaction, which benzene derivative would you expect to react most quickly? Which would react most slowly?
(a)
Problem 62b
For each of the molecules shown, predict the structure of at least one major fragment in the mass spectrum.
(b)
Problem 62d
For each of the molecules shown, predict the structure of at least one major fragment in the mass spectrum.
(d)
Problem 62e
For each of the molecules shown, predict the structure of at least one major fragment in the mass spectrum.
(e)
Problem 64
Give the structure of the molecule that gives rise to the following IR and mass spectra.
<IMAGE>
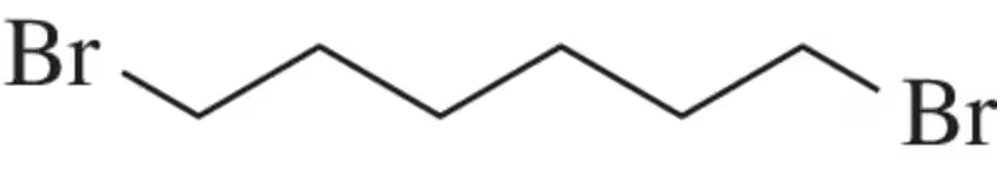
Problem 68
What ratio of M, M + 2 , M + 4 and would you expect for 1,6-dibromohexane?