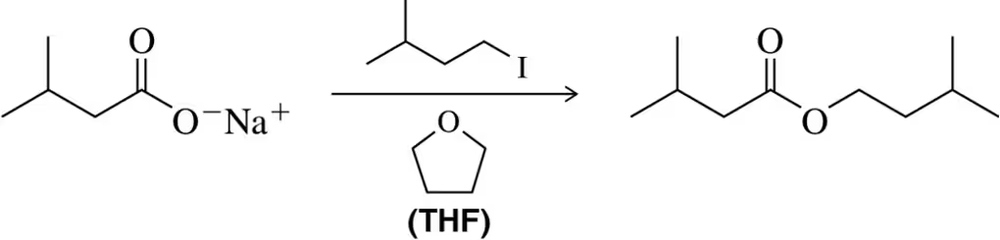
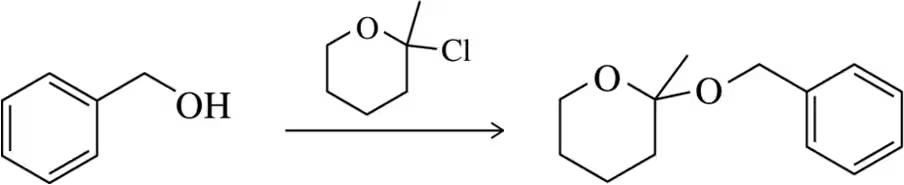
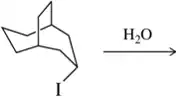
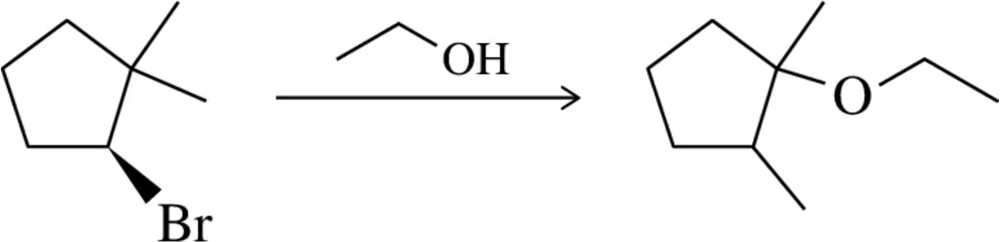
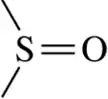Back
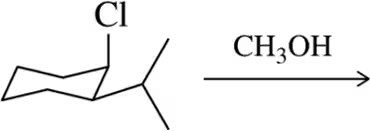
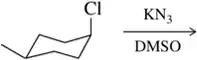
BackProblem 28f
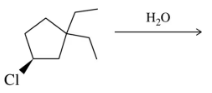
For each solvent, indicate the most likely substitution reaction to take place.
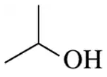
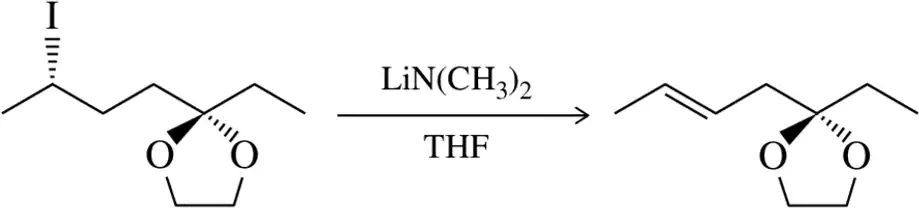
(f)
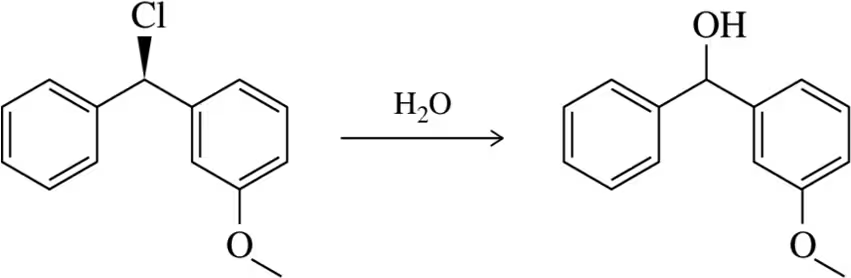
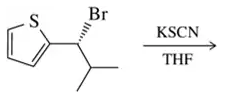
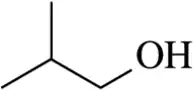
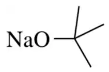
Problem 28g
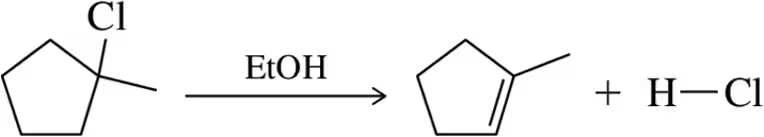
For each solvent, indicate the most likely substitution reaction to take place.
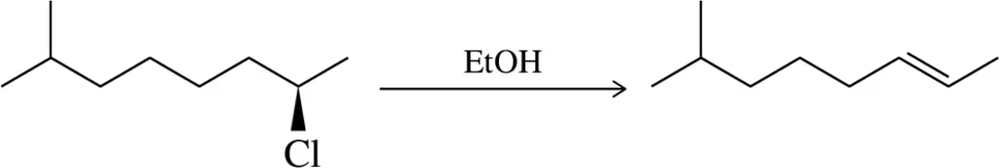
(g)
Problem 29a
Would you expect the following conditions to favor SN1 or SN2?
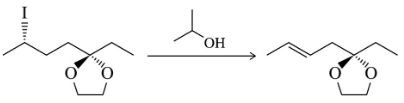
(a)
Problem 29b
Would you expect the following conditions to favor SN1 or SN2?
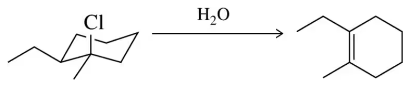
(b)
Problem 29d
Would you expect the following conditions to favor SN1 or SN2?
(d)
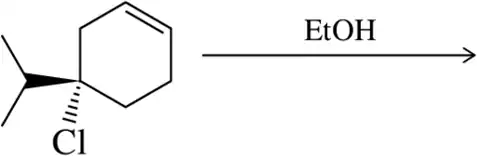
Problem 30a
Suggest a mechanism for the following substitution reactions.
(a)
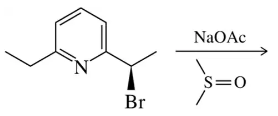
Problem 30b
Suggest a mechanism for the following substitution reactions.
(b)
Problem 30c
Suggest a mechanism for the following substitution reactions.
(c)
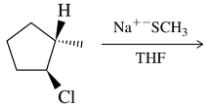
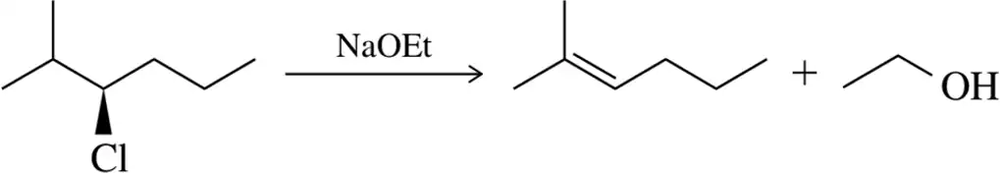
Problem 31a
Predict the product of the substitution reactions, paying attention to the stereochemical outcome.
(a)
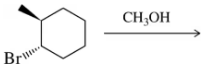
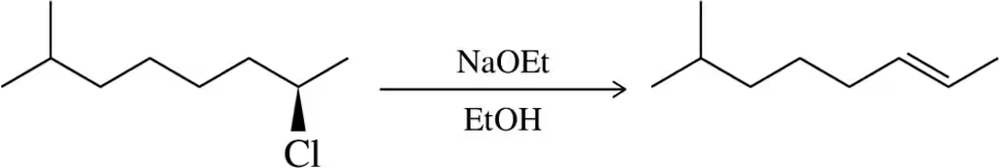
Problem 31b
Predict the product of the substitution reactions, paying attention to the stereochemical outcome.
(b)
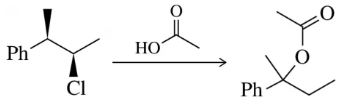
Problem 31d
Predict the product of the substitution reactions, paying attention to the stereochemical outcome.
(d)
Problem 32a
Provide a mechanism for the following SN1 reactions that feature a rearrangement.
(a)
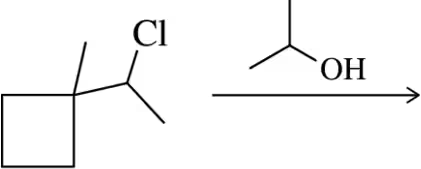
Problem 32b
Provide a mechanism for the following SN1 reactions that feature a rearrangement.
(b)
Problem 33a
Predict the product of the following substitution reactions, making sure to note whether a rearrangement should occur.
(a)
Problem 33b
Predict the product of the following substitution reactions, making sure to note whether a rearrangement should occur.
(b)
Problem 33c
Predict the product of the following substitution reactions, making sure to note whether a rearrangement should occur.
(c)
Problem 33d
Predict the product of the following substitution reactions, making sure to note whether a rearrangement should occur.
(d)
Problem 34a
Identify the bonds that break and form in the following elimination reactions.
(a)
Problem 34b
Identify the bonds that break and form in the following elimination reactions.
(b)
Problem 35
When 2-bromopropane is treated with sodium ethoxide, propene is produced. What molecule is lost from 2-bromopropane in this process?
Problem 36a
(a) How would you convert propene to 2-bromopropane?
Problem 37a
Practice your electron-pushing skills by drawing a mechanism for the following E2 reactions.
(a)
Problem 37b
Practice your electron-pushing skills by drawing a mechanism for the following E2 reactions.
(b)
Problem 38a
Practice your electron-pushing skills by drawing a mechanism for the following E1 reactions.
(a)
Problem 38b
Practice your electron-pushing skills by drawing a mechanism for the following E1 reactions.
(b)
Problem 38c
Practice your electron-pushing skills by drawing a mechanism for the following E1 reactions.
(c)
Problem 39a
Would you expect the following bases to favor E1 or E2 elimination?
(a)
Problem 39c
Would you expect the following bases to favor E1 or E2 elimination?
(c)
Problem 39d
Would you expect the following bases to favor E1 or E2 elimination?
(d) H2O
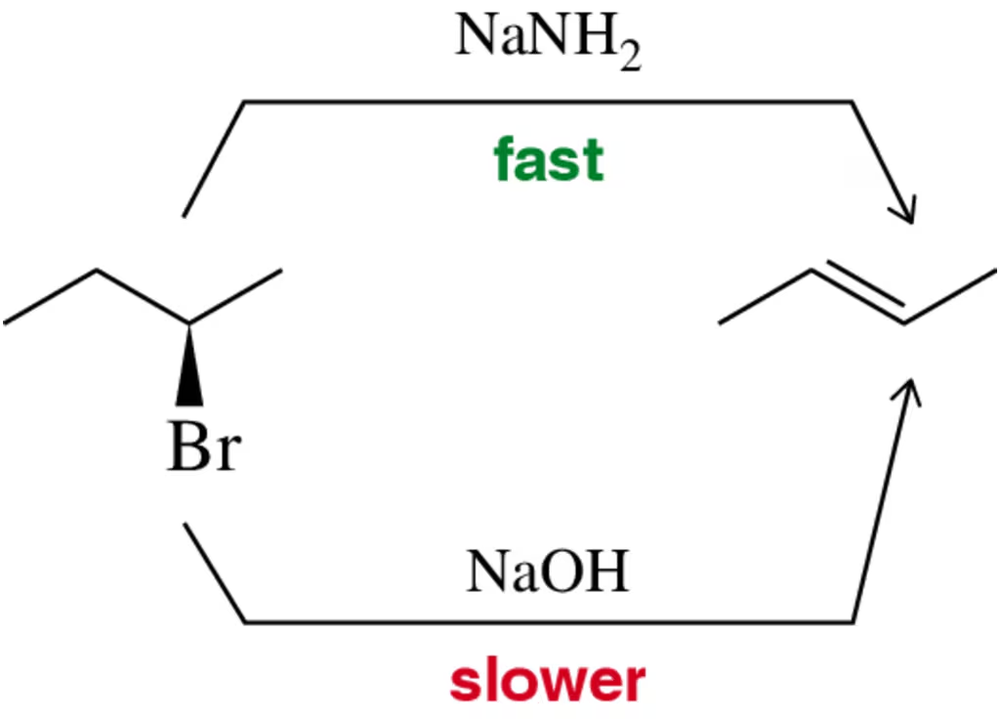
Problem 40
Justify on a reaction coordinate diagram the fact that a strong base like sodium amide (NaNH2) results in a faster E2 elimination than does sodium hydroxide (NaOH).