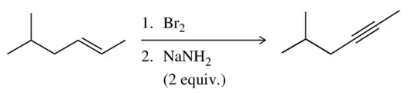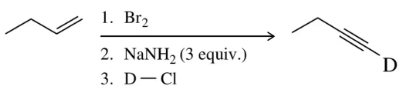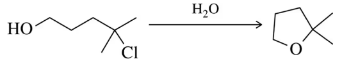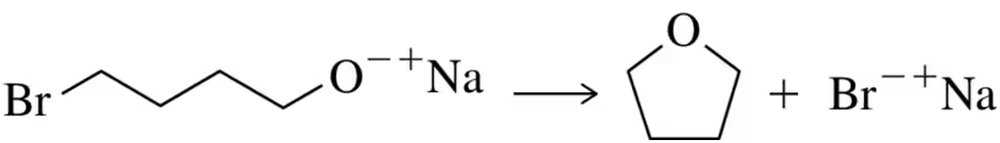Back
BackProblem 61
In Chapter 10, you learned how to make an alkyne by acetylide alkylation with a 1° haloalkane. Suggest a mechanism by which this reaction occurs.
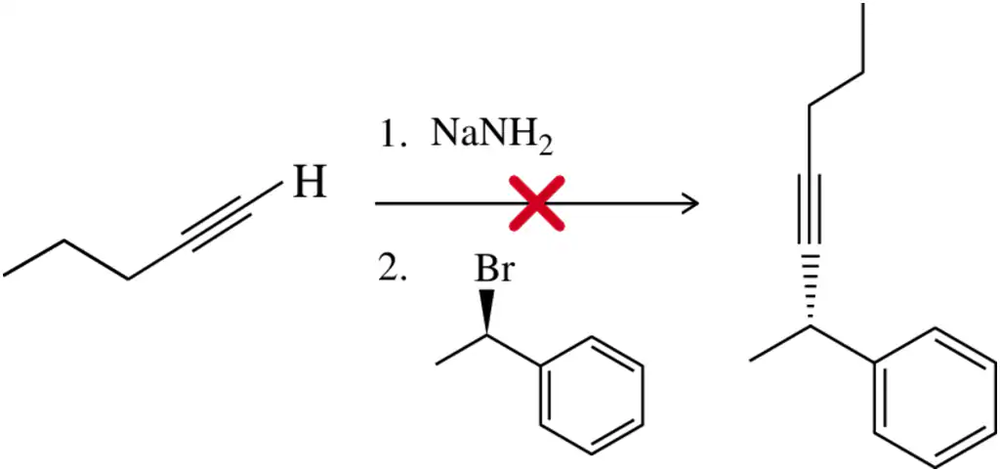
Problem 62
Acetylide alkylation, from Assessment 12.61, fails to give the desired product with 2° haloalkanes. Why? What is the actual product of this reaction?
Problem 63
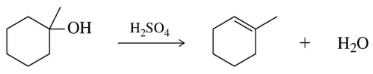
The introduction of elimination reactions provides a second way to synthesize alkynes in a two-step process starting with an alkene. Suggest a mechanism for both steps of this process.
Problem 64
When the reaction scheme in Assessment 12.63 is done on a monosubstituted alkene, at least three equivalents of base are needed. Reacting the product of step 2 with D–Cl (D is an isotope of H) incorporates deuterium at the terminal carbon. Explain these two observations.
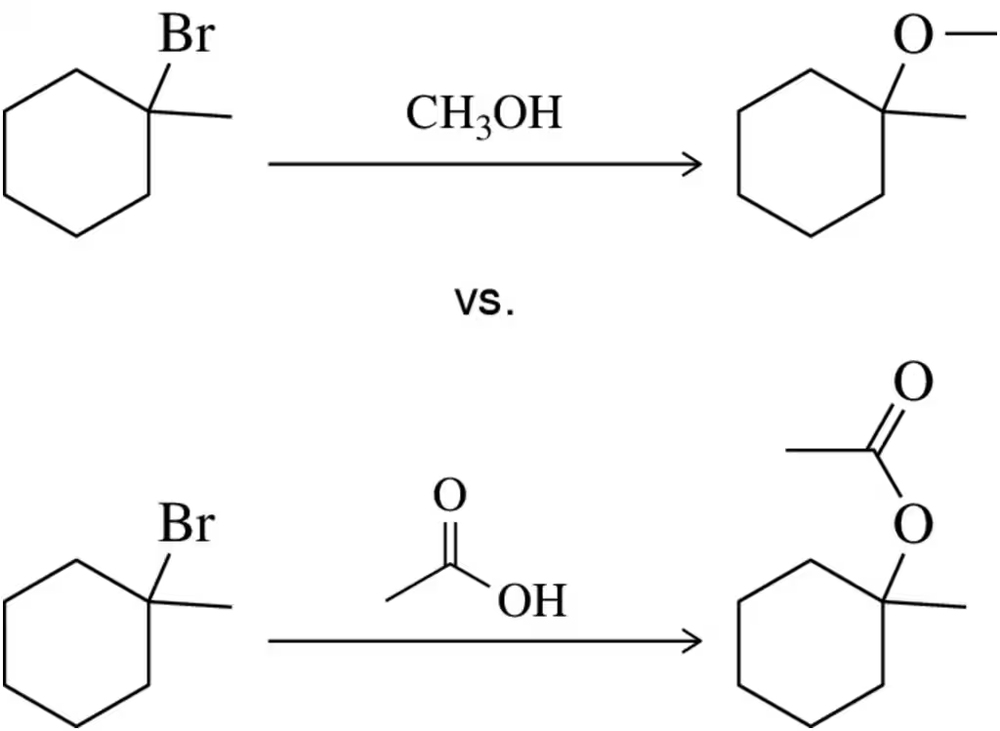
Problem 65
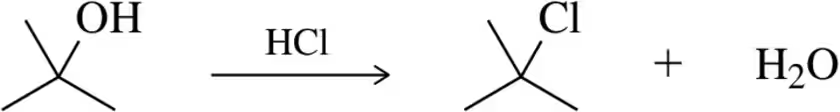
Which of the following substitution reactions would you expect to occur more quickly? Explain your answer.
Problem 66
Give a mechanism for the following substitution and elimination reactions.
(a)
Problem 66b
Give a mechanism for the following substitution and elimination reactions.
(b)
Problem 66c
Give a mechanism for the following substitution and elimination reactions.
(c)
Problem 67
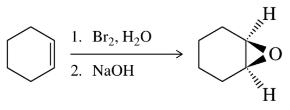
In addition to using mCPBA, epoxides can be synthesized from alkenes in the two-step process shown. Give a mechanism for each step of the process.
Problem 68
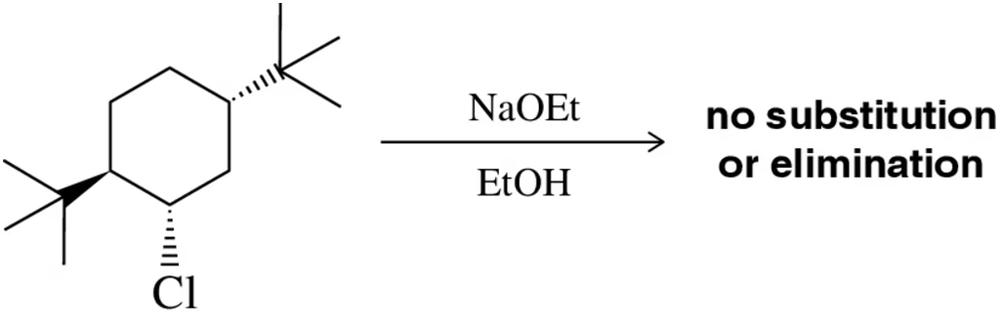
The following chlorocyclohexane undergoes neither Sₙ2 nor E2 under the conditions shown. Why?
Problem 69
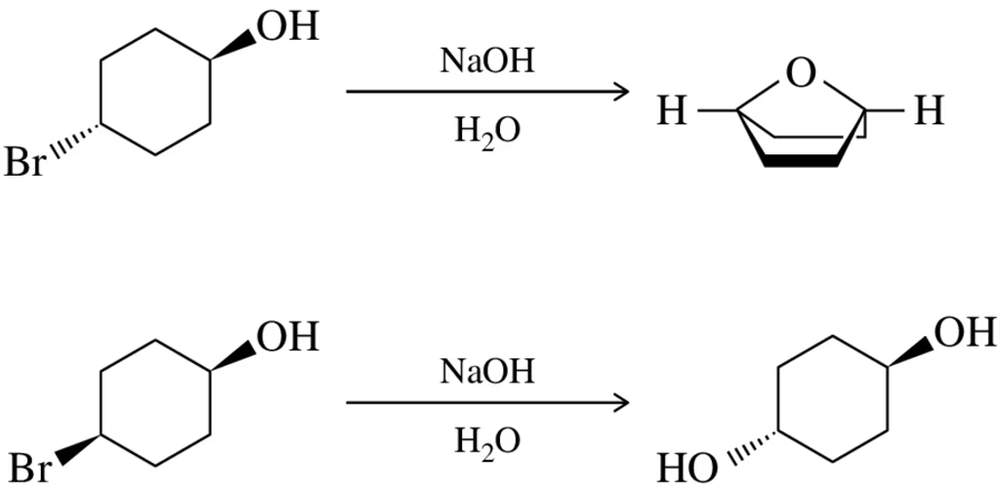
When trans-4-bromocyclohexanol is treated with base, an intramolecular substitution reaction occurs to give a cyclic ether. This product does not form when cis-4-bromocyclohexanol is reacted under the same conditions. Explain these observations.
Problem 70
The following substitution reaction, between a strong base and a 1° haloalkane, occurs in a single step via backside displacement. Yet it is not technically an SN2 reaction. Why?
Problem 71
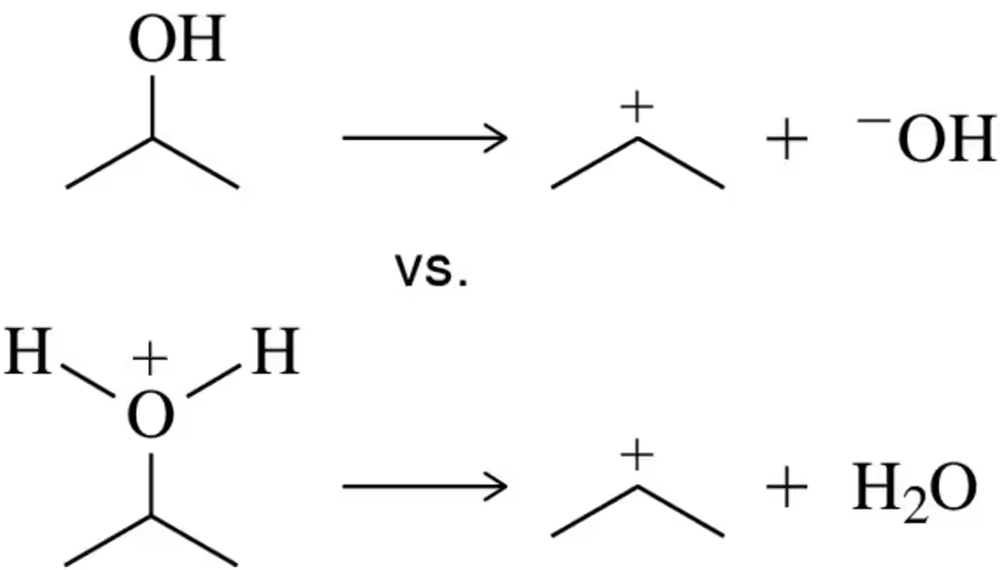
Which is a better leaving group, HO⁻ or H2O? Explain your answer.
Problem 72
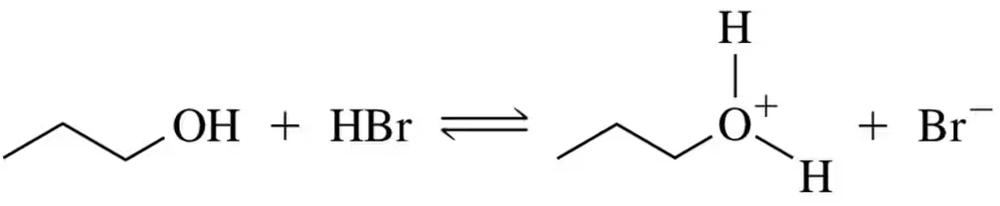
Using pKₐ values, calculate Keq for the following acid–base reaction.
Problem 73a
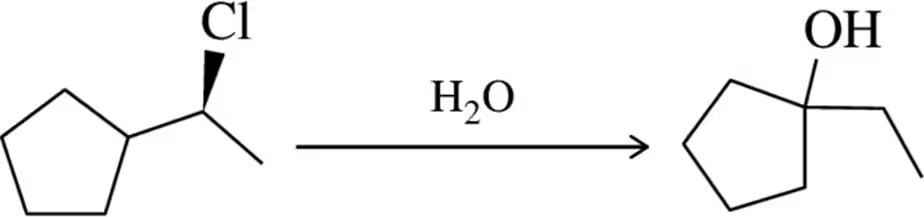
Suggest a mechanism for the following reactions.
(a) Substitution:
Problem 73b
Suggest a mechanism for the following reactions.
(b) Substitution :
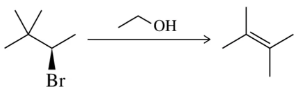
Problem 73c
Suggest a mechanism for the following reactions.
(c) Elimination:
Problem 75
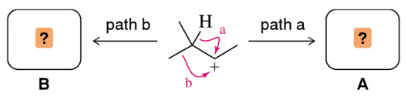
In this, and previous, chapters, we have seen 1,2-alkyl and 1,2-hydride shifts. If both are possible, as in the carbocation shown, which would you expect to occur? Explain your answer.
Problem 77
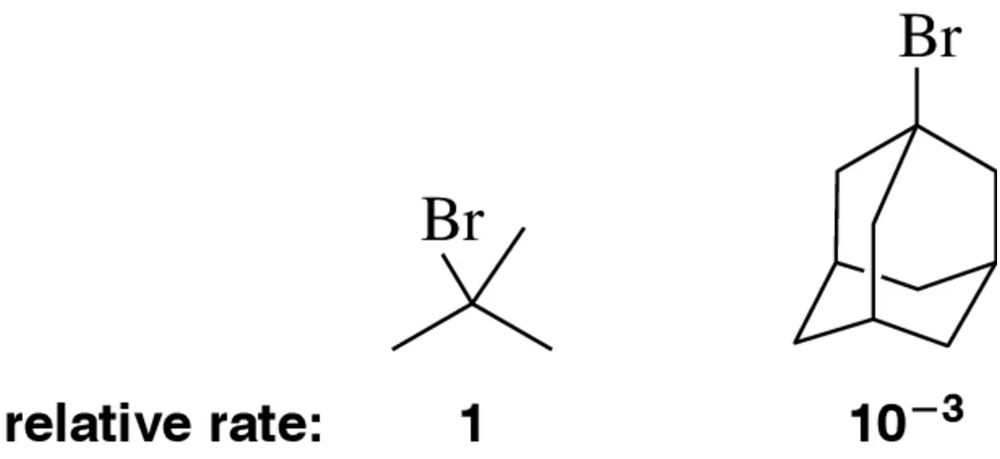
The bromoalkanes shown below participate in SN1 reactions with the relative rates shown. Explain this trend. relative rate:
Problem 78
Of the three possible elimination mechanisms (Figure 12.50), this chapter focused on two of them (E1 and E2). The third possibility occurs in situations like the one below. What makes this mechanism favored under these conditions?
Problem 79
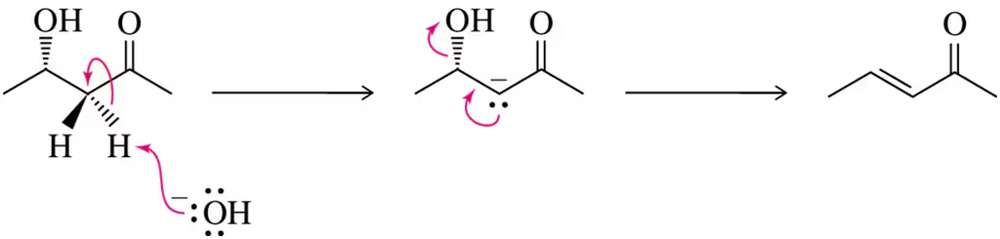
In Chapter 8, we learned about the chemistry of terpenes and the interesting reactions they can undergo. One such reaction is the acid-catalyzed conversion of nerol to terpineol. Suggest a mechanism for this transformation
.
Problem 80
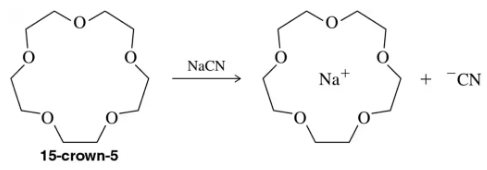
Crown ethers are able to solvate cations based on their size. Specifically, 15-crown-5 forms stable complexes with sodium. How would the addition of a crown ether change the rate of an SN2 reaction?