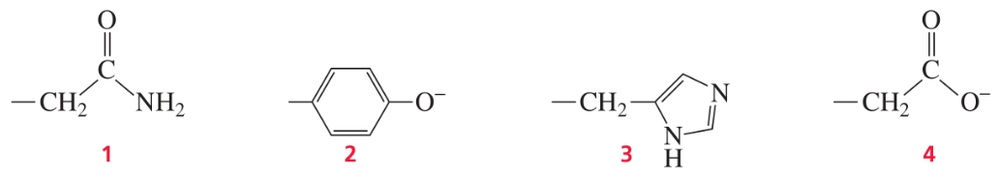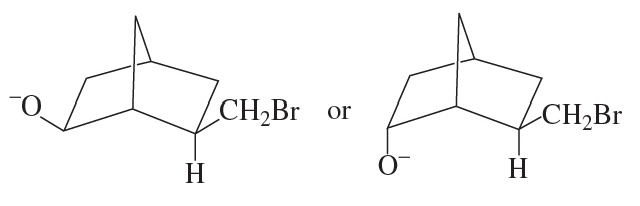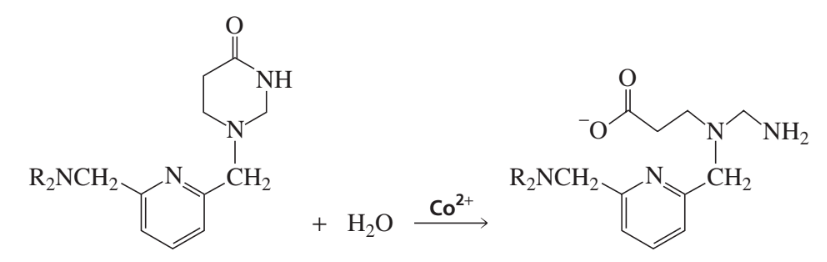Back
BackProblem 2(4)
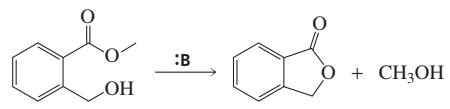
Compare each of the mechanisms listed here with the mechanism for each of the two parts of the acid-catalyzed hydrolysis of an ester, indicating a. similarities. b. differences.
4. acid-catalyzed hydrolysis of an amide
Problem 2(1)
Compare each of the mechanisms listed here with the mechanism for each of the two parts of the acid-catalyzed hydrolysis of an ester, indicating a. similarities. b. differences.
1. acid-catalyzed formation of a hydrate
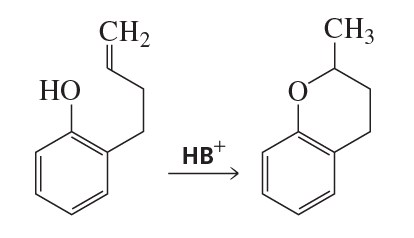
Problem 4a
Draw the mechanism for the following reaction if it involves specific-acid catalysis.
Problem 4b
Draw the mechanism if it involves general-acid catalysis.
Problem 6a
a. Draw the mechanism for the following reaction if it involves specific-base catalysis.
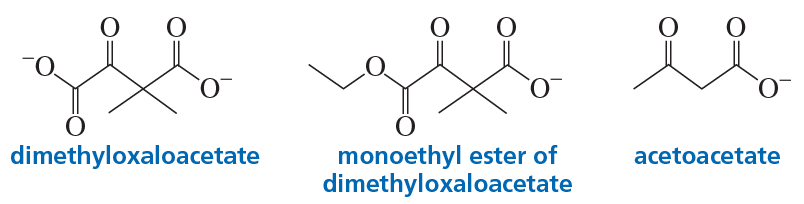
Problem 7
Although metal ions increase the rate of decarboxylation of dimethyloxaloacetate, they have no effect on the rate of decarboxylation of either the monoethyl ester of dimethyloxaloacetate or acetoacetate. Explain why this is so.
Problem 9
The relative rate of reaction for the cis alkene (E) is given in Table 22.2. What do you expect the relative rate of reaction for the trans alkene to be?
Problem 10
Show all the products, including their configurations, that are obtained from the above reaction.
Problem 12
Why do the nitro groups change the relative leaving tendencies of the carboxy and 2,4-dinitrophenoxy groups in the tetrahedral intermediate in Problem 11?
Problem 15
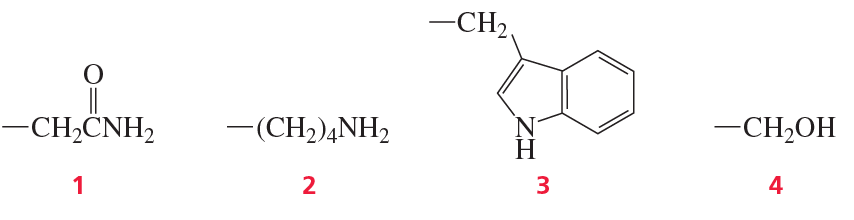
Which of the following amino acid side chains can help remove a proton from the α-carbon of an aldehyde?
Problem 16
Which of the following C-terminal peptide bonds is more readily cleaved by carboxypeptidase A? Explain your choice.
Ser-Ala-Leu or Ser-Ala-Asp
Problem 21a
Draw the pH–activity profile for an enzyme that has one catalytic group at the active site:
a. the catalytic group is a general-acid catalyst with a pKa = 5.6.
Problem 21b
Draw the pH–activity profile for an enzyme that has one catalytic group at the active site:
b. the catalytic group is a general-base catalyst with a pKa = 7.2.
Problem 22
The pH–activity profile for glucose-6-phosphate isomerase indicates the participation of a group with a pKa = 6.7 as a basic catalyst and a group with a pKa = 9.3 as an acid catalyst. Draw the pH–activity profile and identify the amino acids that participate in the catalysis.
Problem 24
Draw the mechanism for the hydroxide ion–catalyzed cleavage of fructose-1,6-bisphosphate.
Problem 26
Which of the following amino acid side chains can form an imine with a substrate?
Problem 27
In glycolysis, why must glucose-6-phosphate isomerize to fructose-6-phosphate (Section 22.12 ) before the cleavage reaction with aldolase occurs?
Problem 28
Aldolase shows no activity if it is incubated with iodoacetic acid before fructose-1,6-bisphosphate is added to the reaction mixture. What causes this loss of activity?
Problem 29
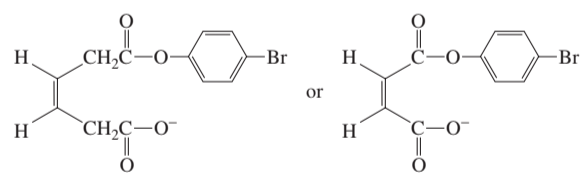
Which of the following two compounds eliminates HBr more rapidly in a basic solution?
Problem 30
Which compound forms an anhydride more rapidly?
Problem 31
Which compound has the greatest rate of hydrolysis at pH = 3.5: benzamide, o-carboxybenzamide, o-formylbenzamide, or o-hydroxybenzamide?
Problem 32a
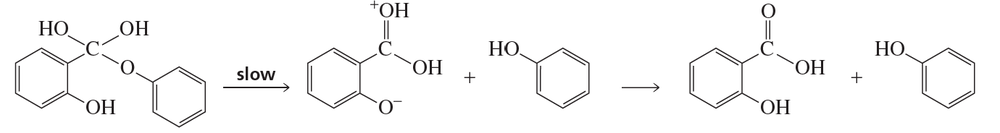
Indicate the type of catalysis that is occurring in the slow step in each of the following reaction sequences:
a.
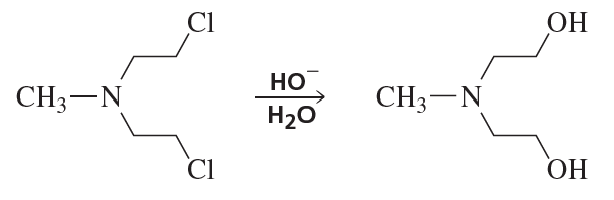
Problem 32b
Indicate the type of catalysis that is occurring in the slow step in each of the following reaction sequences:
b.
Problem 33
The deuterium kinetic isotope effect (kH2O/kD2O) for the hydrolysis of aspirin is 2.2. What does this tell you about the kind of catalysis exerted by the ortho-carboxyl substituent? (Hint: It is easier to break an O–H bond than an O–D bond.)
Problem 34
The rate constant for the uncatalyzed reaction of two molecules of glycine ethyl ester to form glycylglycine ethyl ester is 0.6 M-1 s-1. In the presence of Co2+, the rate constant is 1.5 × 106 M-1 s-1. What rate enhancement does the catalyst provide?
Problem 35
Co2+ catalyzes the hydrolysis of the lactam shown here. Propose a mechanism for the metal-ion catalyzed reaction.
Problem 37
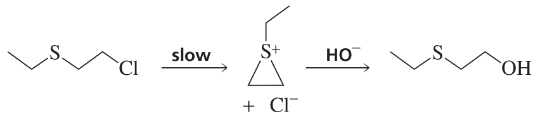
Propose a mechanism for the following reaction. (Hint: The rate of the reaction is much slower if the nitrogen atom is replaced by CH.)
Problem 39
Carbonic anhydrase is an enzyme that catalyzes the conversion of carbon dioxide to bicarbonate ion. It is a metalloenzyme, with Zn2+ coordinated at the active site by three histidine side chains. Propose a mechanism for the reaction.
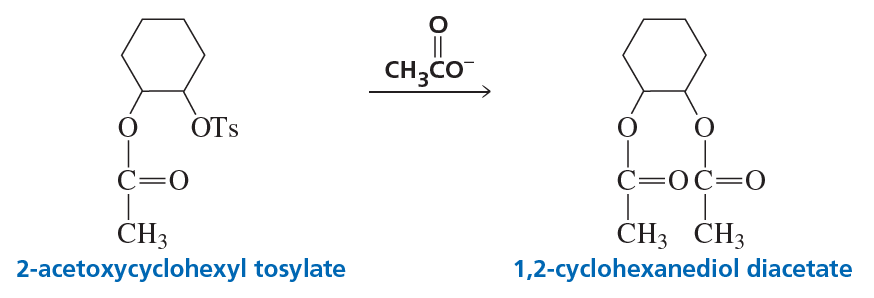
Problem 41a
2-Acetoxycyclohexyl tosylate reacts with acetate ion to form 1,2-cyclohexanediol diacetate. The reaction is stereospecific—that is, the stereoisomers obtained as products depend on the stereoisomer used as a reactant. Recall that because 2-acetoxycyclohexyl tosylate has two asymmetric centers, it has four stereoisomers—two are cis and two are trans. Explain the following observations:
a. Both cis reactants form an optically active trans product, but each cis reactant forms a different trans product.
Problem 41b
2-Acetoxycyclohexyl tosylate reacts with acetate ion to form 1,2-cyclohexanediol diacetate. The reaction is stereospecific—that is, the stereoisomers obtained as products depend on the stereoisomer used as a reactant. Recall that because 2-acetoxycyclohexyl tosylate has two asymmetric centers, it has four stereoisomers—two are cis and two are trans. Explain the following observations:
b. Both trans reactants form the same racemic mixture.