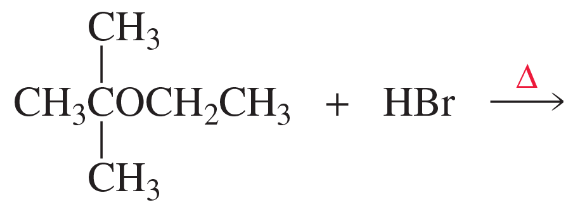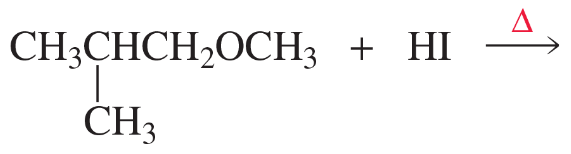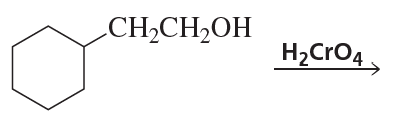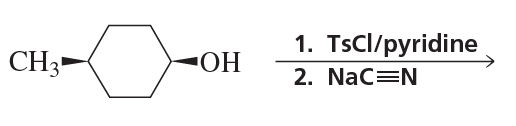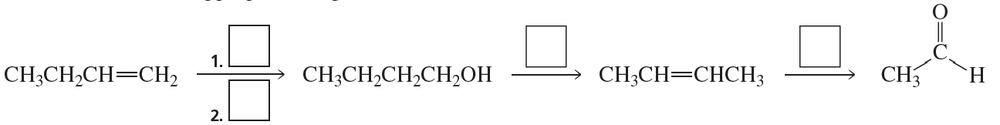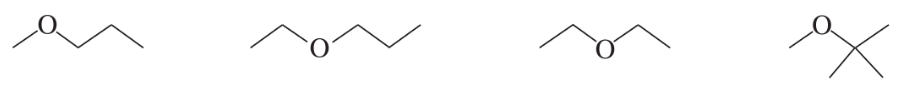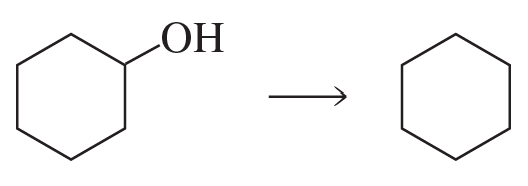Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.10 - Reactions of Alcohols, Ethers, Epoxides, Amines, and Sulfur-Containing Compounds
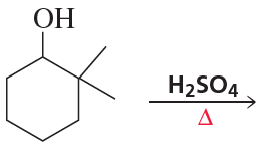
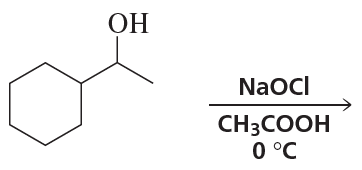
Ch.10 - Reactions of Alcohols, Ethers, Epoxides, Amines, and Sulfur-Containing CompoundsProblem 57a(9)
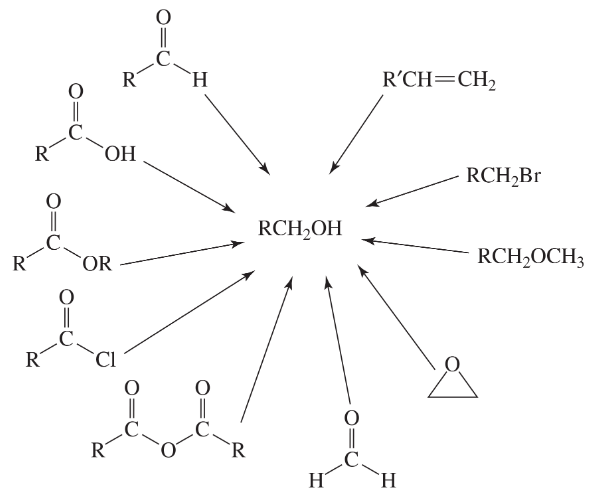
a. Show the reagents required to form the primary alcohol in each of the following reactions.
Problem 58a
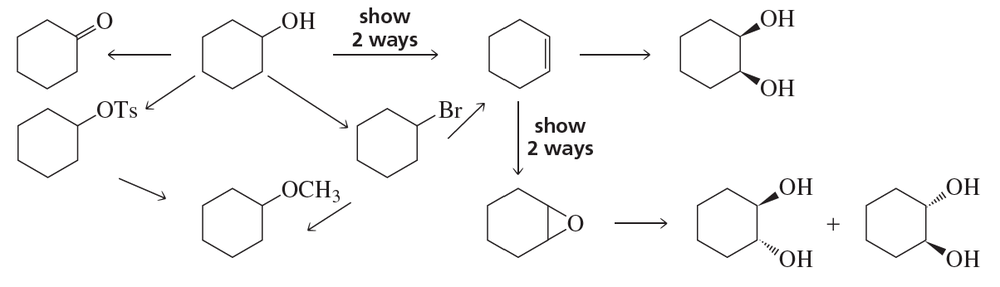
Starting with (R)-1-deuterio-1-propanol, how could you prepare
a. (S)-1-deuterio-1-propanol?
Problem 58b
Starting with (R)-1-deuterio-1-propanol, how could you prepare
b. (S)-1-deuterio-1-methoxypropane?
Problem 58c
Starting with (R)-1-deuterio-1-propanol, how could you prepare
c. (R)-1-deuterio-1-methoxypropane?
Problem 59a
When heated with H2SO4, both 3,3-dimethyl-2-butanol and 2,3-dimethyl-2-butanol are dehydrated to form 2,3-dimethyl-2-butene. Which alcohol dehydrates more rapidly?
Problem 60a,b
What is the major product obtained from the reaction of 2-ethyloxirane with each of the following reagents?
a. 0.1MHCl
b. CH3OH/HCl
Problem 60c
What is the major product obtained from the reaction of 2-ethyloxirane with each of the following reagents?
c. 0.1MNaOH
Problem 60d
What is the major product obtained from the reaction of 2-ethyloxirane with each of the following reagents?
d. CH3OH/CH3O-
Problem 61a
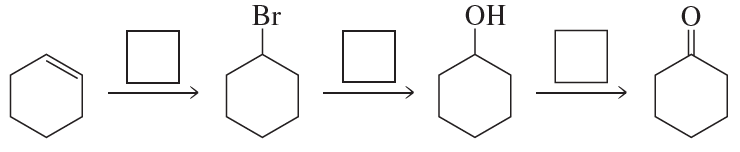
Write the appropriate reagent over each arrow.
Problem 61b
Write the appropriate reagent over each arrow.
Problem 61c
Write the appropriate reagent over each arrow.
Problem 61d
Write the appropriate reagent over each arrow.
Problem 61e
Write the appropriate reagent over each arrow.
Problem 61f
Write the appropriate reagent over each arrow.
Problem 62
What alkenes would you expect to be obtained from the acid-catalyzed dehydration of 1-hexanol?
Problem 63a,b
What is the major product(s) of each of the following reactions?
a.
b.
Problem 63c
What is the major product(s) of each of the following reactions?
c.
Problem 63d
What is the major product(s) of each of the following reactions?
d.
Problem 63g
What is the major product(s) of each of the following reactions?
g.
Problem 63h
What is the major product(s) of each of the following reactions?
h.
Problem 64b
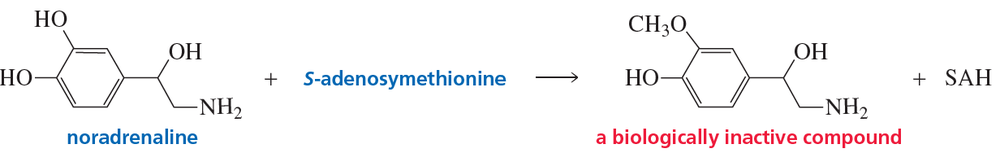
In Section 10.12, we saw that S-adenosylmethionine (SAM) methylates the nitrogen atom of noradrenaline to form adrenaline, a more potent hormone. If SAM methylates an OH group attached to the benzene ring instead, it completely destroys noradrenaline's activity.
b. Which reaction is more apt to occur, methylation on nitrogen or methylation on oxygen?
Problem 66
An unknown alcohol with a molecular formula of C7H14O was oxidized to an aldehyde with HOCl. When an acidic solution of the alcohol was distilled, two alkenes were obtained. The alkene formed in greater yield was determined to be 1-methylcyclohexene. The other alkene formed the original un-known alcohol when treated with BH3/THF followed by H2O2, HO-, and H2O. Identify the unknown alcohol.
Problem 67
Explain why the acid-catalyzed dehydration of an alcohol is a reversible reaction, whereas the base-promoted dehydrohalogenation of an alkyl halide is an irreversible reaction.
Problem 68
Explain why (S)-2-butanol forms a racemic mixture when it is heated in sulfuric acid.
Problem 69a
Fill in each box with the appropriate reagent:
a.
Problem 69c
Fill in each box with the appropriate reagent:
c.
Problem 70(8)
a. Draw the product or products that will be obtained from the reaction of cis-2-butene and trans-2-butene with each of the following reagents. If a product can exist as stereoisomers, show which stereoisomers are formed.
8. CH3OH + H2SO4
b. With which reagents do the two alkenes react to form different products?
Problem 71
What product would be formed if the four-membered ring alcohol in Problem 70 were heated with an equivalent amount of HBr rather than with a catalytic amount of H2SO4?
Problem 72
Which of the following ethers would be obtained in greatest yield directly from alcohols?
Problem 73a
Using the given starting material, any necessary inorganic reagents, and any carbon-containing compounds with no more than two carbons, indicate how the following syntheses could be carried out:
a.