Osmosis is a vital biological process defined as the passive diffusion of water across a semipermeable membrane, such as a cell membrane, without the need for energy. This process is crucial for maintaining cellular homeostasis and is influenced by the tonicity of the surrounding solutions. Tonicity refers to the relative concentration of solutes in a solution compared to another solution, typically the fluid inside a cell.
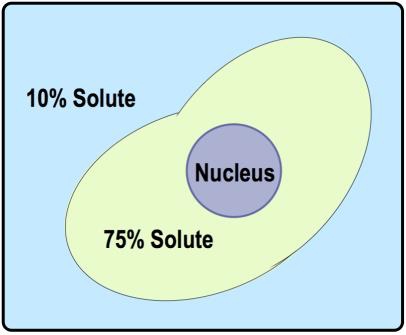
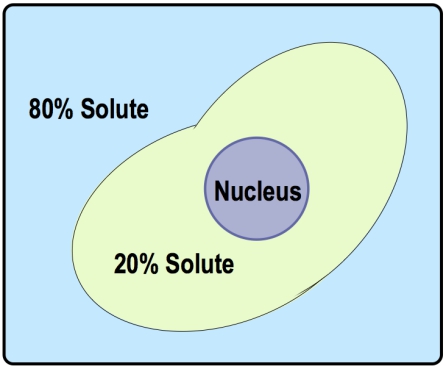
There are three key terms associated with tonicity: hypotonic, isotonic, and hypertonic. A hypotonic solution has a lower concentration of solutes compared to another solution, leading to water moving into the cell, which can cause the cell to swell. Conversely, a hypertonic solution has a higher concentration of solutes, resulting in water moving out of the cell, which can cause the cell to shrink. An isotonic solution has equal concentrations of solutes, meaning there is no net movement of water, and the cell remains stable in size.
Understanding these terms requires comparing two regions: the inside of the cell and the surrounding solution. For example, if the outside solution has fewer solutes than the inside, it is labeled as hypotonic. If the concentrations are equal, it is isotonic, and if the outside has more solutes, it is hypertonic. This comparative analysis is essential for predicting the direction of water flow during osmosis.
In summary, osmosis is a passive process driven by the differences in solute concentrations across membranes, and understanding tonicity is crucial for grasping how cells interact with their environments. This foundational knowledge sets the stage for exploring the dynamics of water movement in biological systems.