Hydrogen bonding, often abbreviated as H bond, is a crucial interaction in chemistry and biology. It occurs between a hydrogen atom and a highly electronegative atom, typically fluorine, oxygen, or nitrogen. To remember these electronegative atoms, think of the acronym "FON," which sounds like "fun." Each hydrogen bond involves a hydrogen atom paired with one of these electronegative atoms, which are essential for various biological processes.
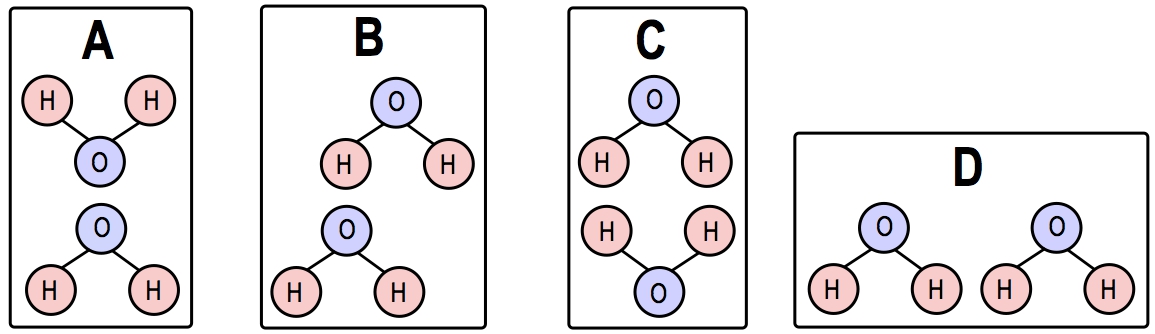
Individually, hydrogen bonds are relatively weak; however, when numerous hydrogen bonds form collectively, they can create significant strength. This property is particularly important in biological systems, such as the unique characteristics of water and the structure of macromolecules. For instance, water molecules (H2O) can form hydrogen bonds with each other, where the hydrogen atom of one water molecule interacts with the highly electronegative oxygen atom of another. This bonding is vital for the unique properties of water that support life.
Additionally, hydrogen bonds play a significant role in the structure of nucleotides, the building blocks of DNA. In nucleotides, hydrogen bonds can form between a hydrogen atom and either an oxygen or a nitrogen atom, facilitating the stability of the DNA structure. Understanding hydrogen bonding is essential as it underpins many biological functions and molecular interactions, setting the stage for deeper exploration of these concepts in future studies.