3. Energy & Cell Processes
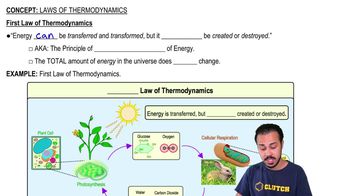
Laws of Thermodynamics
Learn with other creators
Practice this topic
- Multiple Choice
Which of the following statements describes the first law of thermodynamics?
a) Energy cannot be created or destroyed.
b) Energy cannot be transferred or transformed.
c) Also called The Principle of Creation of Energy.
d) Energy can be destroyed.
- Multiple Choice
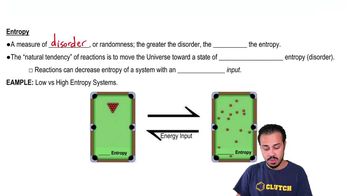
Which of the following images has less entropy?
a) Image A has less entropy.
b) Image B has less entropy.
- Multiple Choice
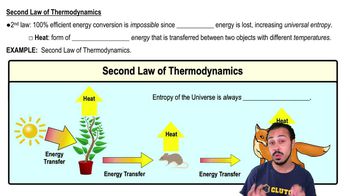
When chemical, transport, or mechanical work is done by an organism, what happens to the heat generated?
a) It is used to power yet more cellular work in the surroundings.
b) It is captured to store energy as more heat in the system.
c) It is used to generate ADP.
d) It is lost to the environment.
- Multiple Choice
Which of the following statements is true regarding how energy moves up the food chain?
a) All of the energy is not transferred from producer to consumer because some of the energy is destroyed.
b) All of the energy is transfer from producer to consumer.
c) All of the energy is not transferred from producer to consume because some of the energy is lost as heat.
d) None of the above.
- Open QuestionRelate the laws of thermodynamics to living organisms.
- Open Question
Match the following terms with the correct definition:
_____Endergonic reaction a. Energy in motion
_____Potential energy b. Energy stored in chemical bonds
_____Electrical energy c. Reaction that consumes energy
_____Anabolic reaction d. A decomposition reaction
_____Oxidation-reduction reaction e. The energy of moving charged particles
_____Chemical energy f. Stored energy
_____Catabolic reaction g. Synthesis reaction
_____Kinetic energy h. Reaction where electrons are transferred between reactants