2. Cell Chemistry & Cell Components
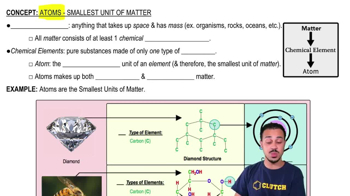
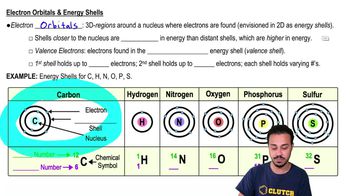
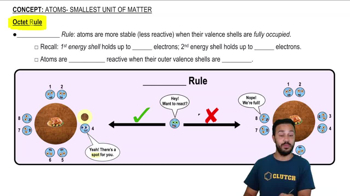
Atoms- Smallest Unit of Matter
2. Cell Chemistry & Cell Components
Atoms- Smallest Unit of Matter
Showing 9 of 9 videos
Additional 3 creators.
Learn with other creators
Practice this topic
- Multiple Choice
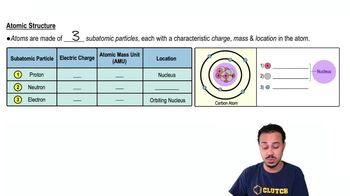
A proton ___________:
a) Has one positive charge.
b) Has one AMU.
c) Is found in the nucleus of the atom.
d) Only a and b are true.
e) a, b, and c are true.
- Multiple Choice
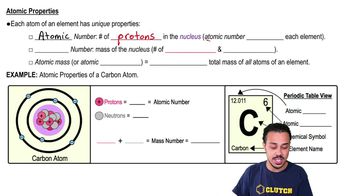
The average oxygen atom has a mass number of 16 and an atomic number of 8. This means that the number of neutrons in this oxygen atom is:
a) 24.
b) 8.
c) 16.
d) 4.
e) 2.
- Multiple Choice
How many valence electrons does an atom with five total electrons have?
a) 5.
b) 7.
c) 3.
d) 2.
e) 1.
- Multiple Choice
Which of the following is true about electron energy shells?
a) They represent regions around the nucleus in which the electrons orbit.
b) The shells closest to the nucleus contain electrons with higher energy.
c) They contain electrons of the same energy.
d) a and b only.
e) a and c only.
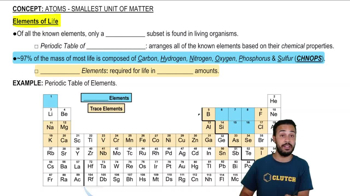
- Open QuestionIn the term trace element, the adjective trace means thata. the element is required in very small amounts.b. the element can be used as a label to trace atoms through an organism's metabolism.c. the element is very rare on Earth.d. the element enhances health but is not essential for the organism's long-term survival.2views
- Open QuestionFill in the blanks in this concept map to help you tie together the key concepts concerning elements, atoms, and molecules.2views
- Open QuestionWe can be sure that a mole of table sugar and a mole of vitamin C are equal in theira. mass.b. volume.c. number of atoms.d. number of molecules.2views
- Open QuestionThe reactivity of an atom arises froma. the average distance of the outermost electron shell from the nucleus.b. the existence of unpaired electrons in the valence shell.c. the sum of the potential energies of all the electron shells.d. the potential energy of the valence shell.2views