2. Cell Chemistry & Cell Components
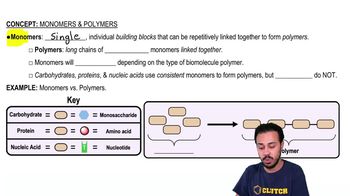
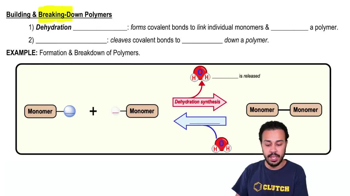
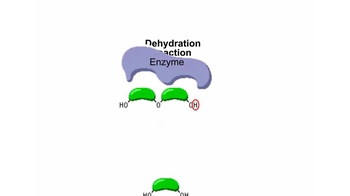
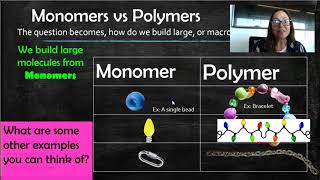
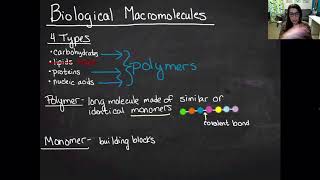
Monomers & Polymers
2. Cell Chemistry & Cell Components
Monomers & Polymers
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
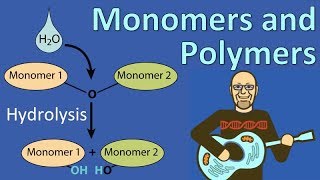
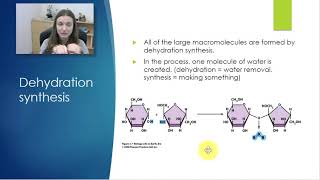
Which of the following statements concerning dehydration reactions and hydrolysis is correct?
a) Dehydration reactions allow solutions to evaporate; hydrolysis reactions dissolve solutes.
b) Dehydration reactions and hydrolysis reactions assemble polymers from monomers.
c) Hydrolysis reactions create polymers from monomers; and dehydration reactions create monomers from polymers.
d) Dehydration reactions create polymers from monomers; hydrolysis reactions break down polymers.
- Multiple Choice
_________ bonds are formed between monomers to form a polymer.
a) Ionic bonds.
b) Covalent bonds.
c) Hydrogen bonds.
d) Hydrophobic bonds.
e). Nuclear bonds.
- Multiple ChoiceWhat is the process by which monomers are linked together to form polymers?
- Multiple ChoiceIn a hydrolysis reaction, __________, and in this process, water is __________.
- Open QuestionThe molecular formula for glucose is C6H12O6. What would be the molecular formula for a polymer made by linking ten glucose molecules together by dehydration reactions?a. C60H120O60b. C60H102O51c. C60H100O50d. C60H111O51