2. Cell Chemistry & Cell Components
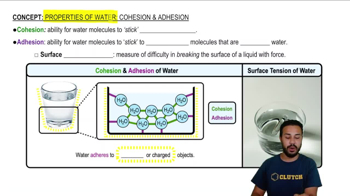
Properties of Water- Cohesion and Adhesion
Learn with other creators
Practice this topic
- Multiple Choice
Which of the following effects can occur because of the high surface tension of water?
a) Lakes cannot freeze solid in winter even with extremely low temperatures.
b) A spider can walk across the surface of a small pond.
c) Organisms can resist temperature changes, although they give off heat due to chemical reactions.
d) Sweat can evaporate from the skin, helping to keep people from overheating.
- Multiple Choice
Cohesive forces in liquid water occur when:
a) The H atoms on molecules of H2O hydrogen bond to O atoms on adjacent molecules of H2O.
b) The H atoms on molecules of H2O hydrogen bond to other H atoms on adjacent molecules of H2O.
c) The atoms on molecules of H2O hydrogen bond to other O atoms on adjacent molecules of H2O.
d) None of the above are correct.
- Multiple ChoiceThe tendency of water molecules to stay close to each other as a result of hydrogen bonding __________.
- Multiple ChoiceWhat do cohesion, surface tension, and adhesion have in common with reference to water?
- Open Question
Which of the following is not a property of water?
a. Water has a high heat capacity.
b. Water doesn't take heat with it when it evaporates.
c. Water is a polar solvent in which many solutes will dissolve.
d. Water serves as a cushion and lubricant in the body.
- Open Question
Which of the following is not a property of water?
a. Water has a high heat capacity.
b. Water doesn't take heat with it when it evaporates.
c. Water is a polar solvent in which many solutes will dissolve.
d. Water serves as a cushion and lubricant in the body.