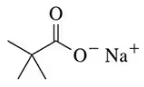
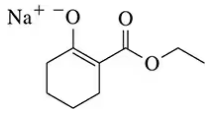
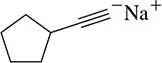
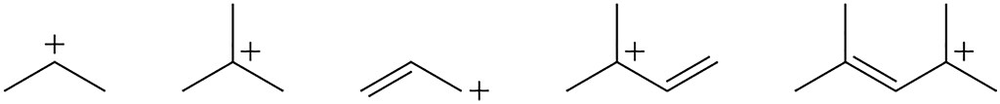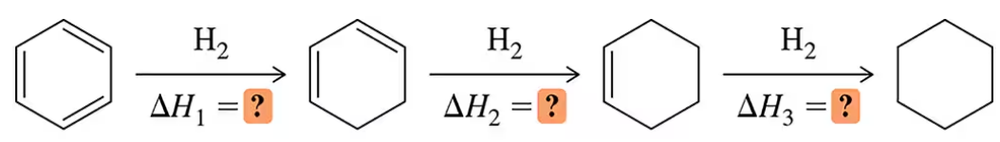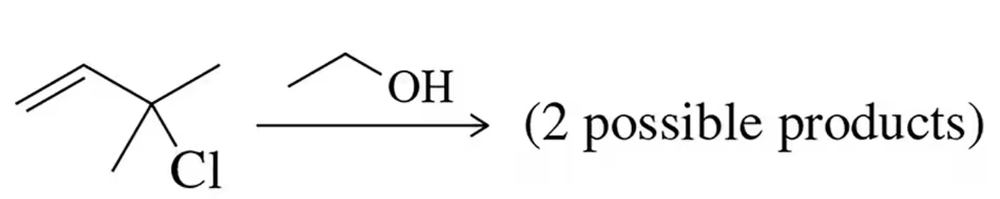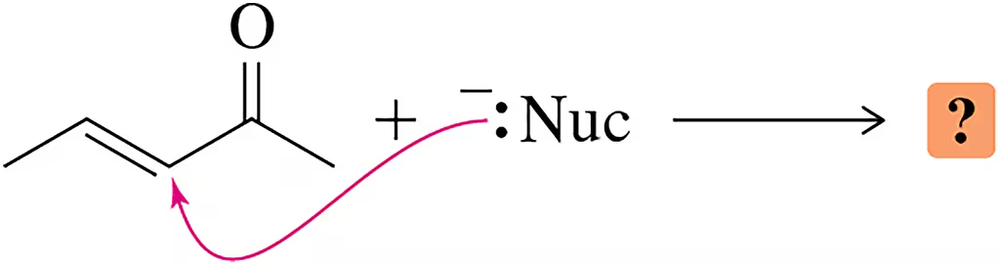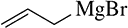Back
BackProblem 1
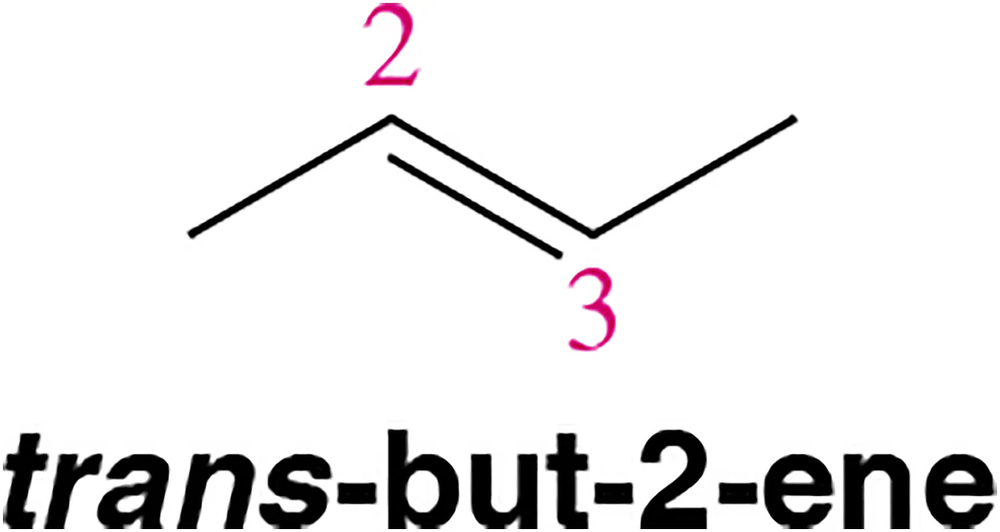
Draw the molecular orbital picture of trans-but-2-ene. Be sure to label all σ and π bonds. Is there free rotation around the C₂― C₃ bond? Why or why not?
Problem 2b
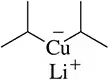
Draw all possible resonance structures for the reactive intermediates shown.
(b)
Problem 2c
Draw all possible resonance structures for the reactive intermediates shown.
(c)
Problem 3
Rank the following carbocations in order of stability (1 = most stable; 5 = least stable ). Explain your order.
Problem 4
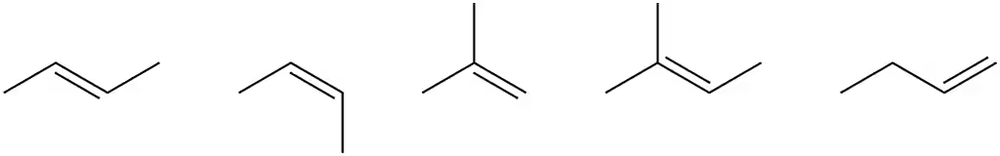
Rank the following alkenes in order of stability (1 = most stable; 5 = least stable). Explain your order.
Problem 5
Predict the product and provide a mechanism for the reaction of 1-methylcyclohexene with HBr.
\
Problem 7
To this point, hydrogenation has always been an exothermic process. Using the numbers from Figure 21.6, calculate ∆Hohydr for each step of the reduction of benzene.
∆H1 + ∆H2 + ∆H3 = ∆Htot = ―49.5 kcal /mol (―208 kJ/mol)
Problem 7a
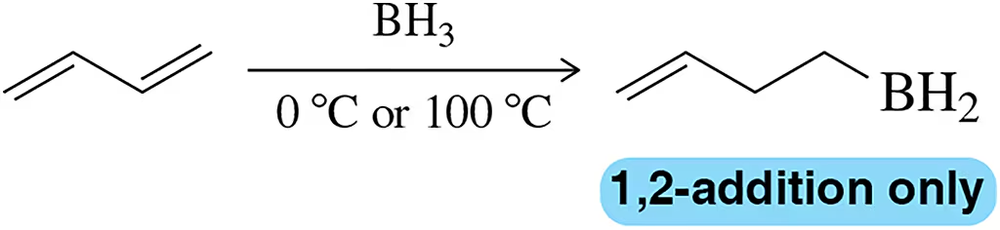
Hydroboration, an electrophilic addition reaction like those studied in Section 21.4, only gives 1,2-addition to buta-1,3-diene, regardless of temperature. Why?
Problem 9
There were actually two possible products in the solvolysis reaction from Figure 21.10. Show both products. Which would you expect to be more stable? Why?
Problem 11
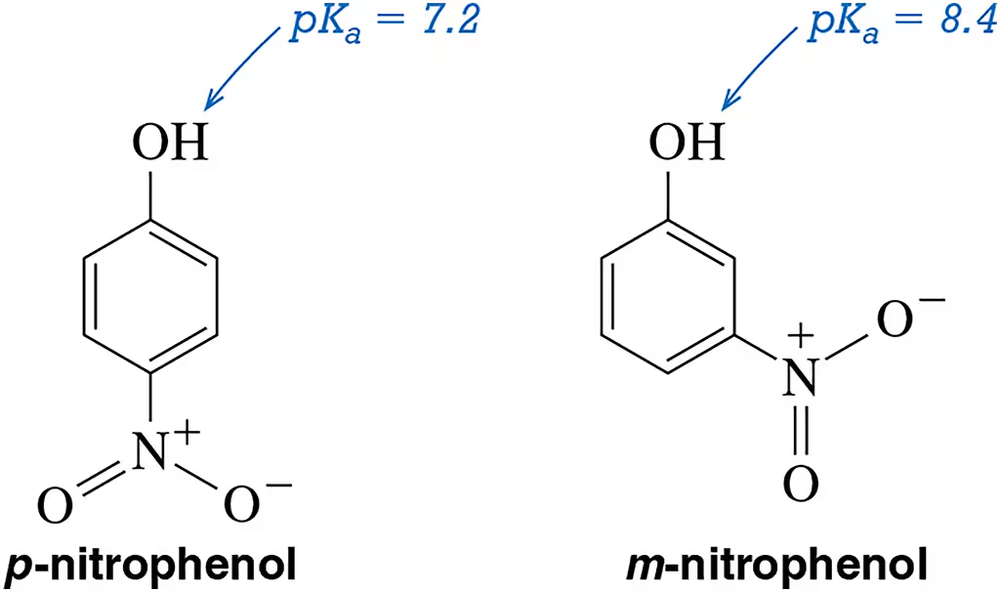
p-Nitrophenol (pKa = 7.2) is ten times more acidic than m-nitrophenol (pKa = 8.4) Explain.
Problem 12
Suppose a molecule is formed by the overlap of 16 atomic orbitals.
(a) How many molecular orbitals will be present?
(b) How many will be bonding?
(c) How many will be antibonding?
Problem 14
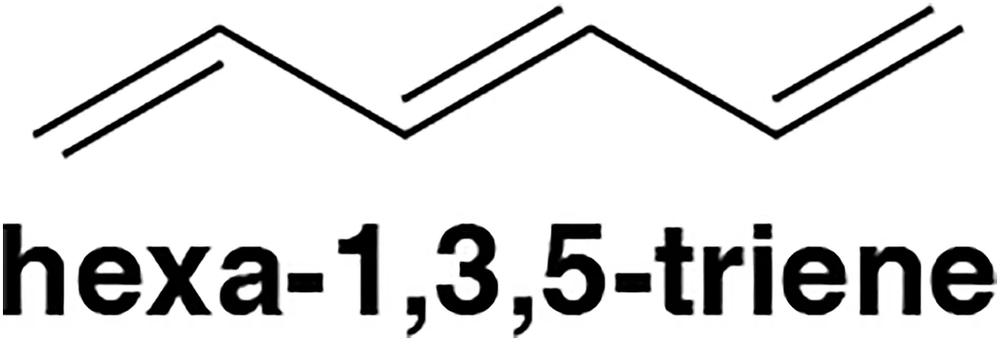
Hexa-1,3,5-triene uses six p orbitals, each containing a single electron, to make its three π bonds. How many total molecular orbitals are made by the six p orbitals?
Problem 15
The molecular orbital picture of H2 can be represented by the following diagram. Label σ and σ* on the diagram—that is, which is (a) and which is (b)? Which is lower in energy? Why?
<IMAGE>
Problem 16
Why is this not a viable representation of the ψ2, molecular orbital of buta-1,3-diene?
Problem 17
The ψ2 molecular orbital for octa-1,3,5,7-tetraene is shown. Draw ψ3.
<IMAGE>
Problem 18
Which of the following would you expect to be a more stable molecular orbital? Why?
<IMAGE>
Problem 20
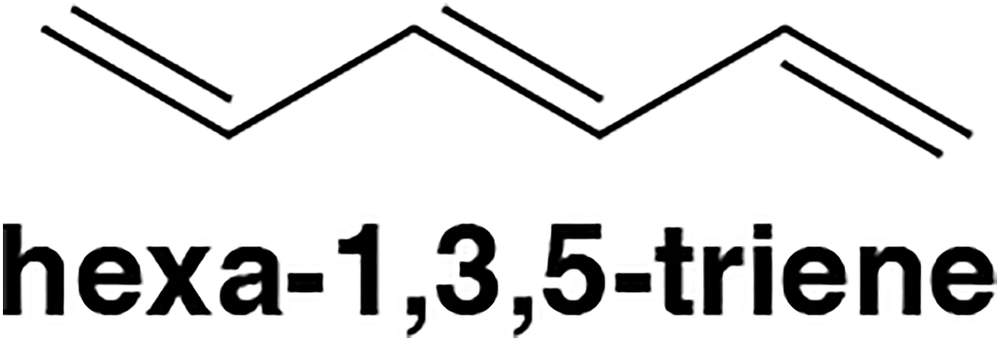
Using the rules described in Section 21.3, draw the molecular orbital picture of hexa-1,3,5-triene. Label the HOMO and LUMO.
- Would you expect the following nucleophiles to do 1,2- or 1,4-addition? a. b. c.
Problem 21
- Would you expect the following nucleophiles to do 1,2- or 1,4-addition? d. e. f.
Problem 21
Problem 21e
Which of the following molecules would you expect to absorb in the visible region of the electromagnetic spectrum?
(e)
Problem 25
Identify the HOMO and LUMO of the allylic cation and the allylic anion shown in Figure 21.22.
<IMAGE>
Problem 27a
Predict the product of the following addition reactions to dienes.
(a)
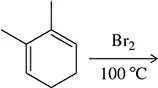
Problem 27c
Predict the product of the following addition reactions to dienes.
(c)
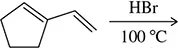
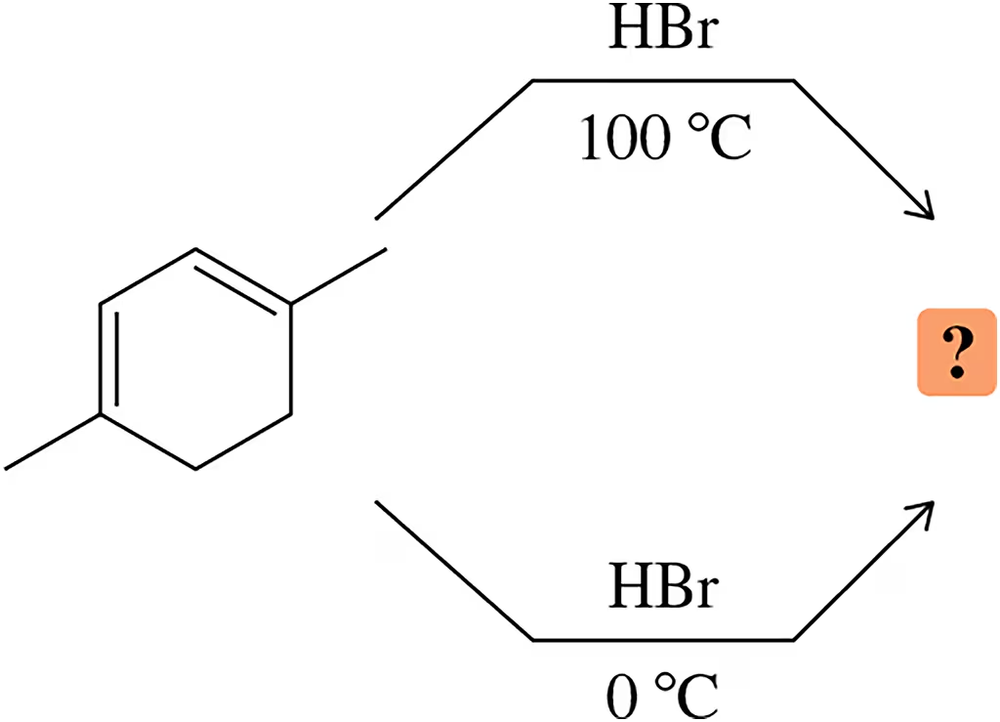
Problem 29
The following diene gives the same product regardless of whether the reaction is run under conditions of kinetic (0 °C) or thermodynamic (100 °C) control. Predict the product and explain this observation.
Problem 32
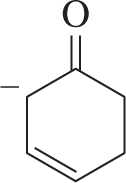
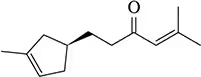
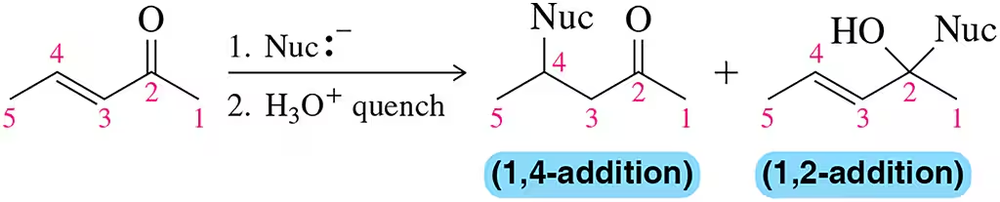
Nucleophilic addition to the α,β-unsaturated ketone shown can occur at either C₂ or C₄. Why?
Problem 33
a. Could a nucleophile ever add to C₃?
b. Why or why not?
Problem 36a,b,c
Would you expect the following nucleophiles to do 1,2- or 1,4-addition?
(a)
(b)
(c) HO–
Problem 36d,e,f
Would you expect the following nucleophiles to do 1,2- or 1,4-addition?
(d)
(e)
(f)
Problem 36g,h,i
Would you expect the following nucleophiles to do 1,2- or 1,4-addition?
(g)
(h)
(i)
Problem 37
Whereas stabilized enolates do 1,4-addition, unstabilized (normal) enolates can do both 1,2- and 1,4-addition depending on the situation. Why might this be?