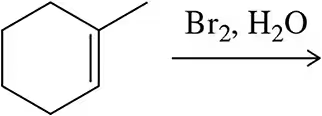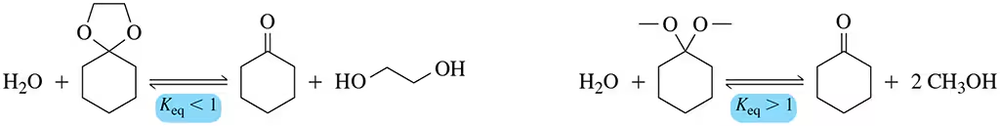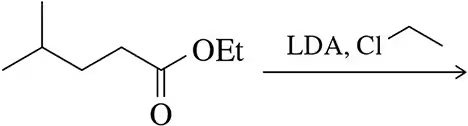Back
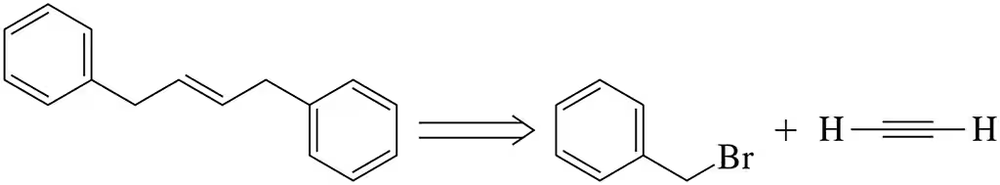
BackProblem 1
Beginning with acetylene and benzyl bromide and using any other inorganic reagents, propose a synthesis of the alkene shown here.
Problem 6a
Predict the product of the following reactions.
(a)
Problem 8
Rationalize the difference in Kₑq for the following reactions. Be sure to account for both ∆S and ∆H.
Problem 15a
Which of the following SN2 and E2 reactions, respectively, is faster? Justify your choice.
(a)
Problem 15b
Which of the following SN2 and E2 reactions, respectively, is faster? Justify your choice.
(b)
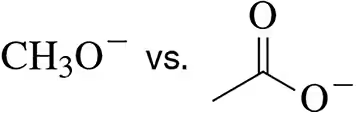
Problem 17b
Specify which in each pair is the harder Lewis base. Justify your choice beyond just looking at Table 20.2.
(b)
Problem 17c
Specify which in each pair is the harder Lewis base. Justify your choice beyond just looking at Table 20.2.
(c) I- vs. F-
Problem 18a
Specify which in each pair is the harder Lewis acid. Justify your choice beyond just looking at Table 20.2.
(a) Al3+ vs. B3+
Problem 18b
Specify which in each pair is the harder Lewis acid. Justify your choice beyond just looking at Table 20.2.
(b) Li+ vs. Na+
Problem 18c
Specify which in each pair is the harder Lewis acid. Justify your choice beyond just looking at Table 20.2.
(c) Mg2+ vs. Na+
Problem 21a
LDA can be used to form enolates on esters and nitriles. Predict the product of these alkylation reactions.
(a)
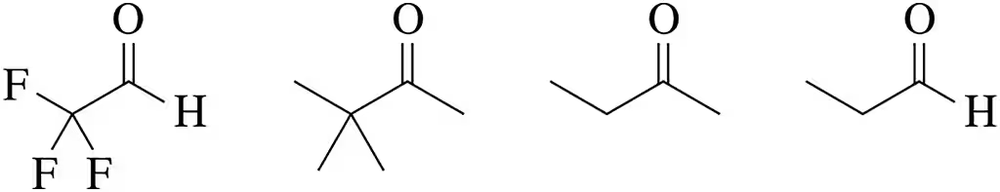
Problem 32
Rank the reactivity of the following carbonyls with nucleophiles, from least reactive to most reactive.
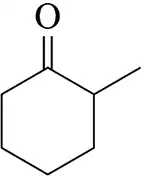
Problem 72a
Identify the enolate(s) that would form on treatment of each of the following carbonyls with base. [When there are two possibilities, draw both.]
(a)