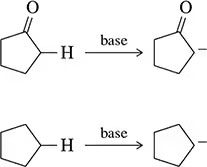
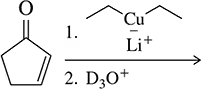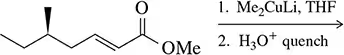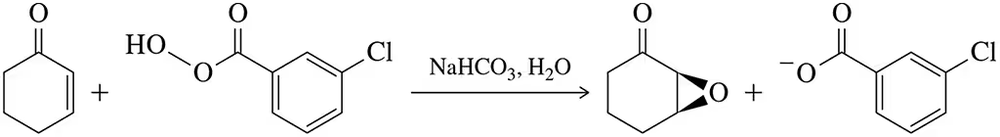Back
BackProblem 43a
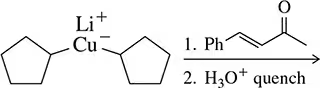
Predict the product of the following reactions.
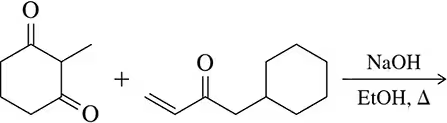
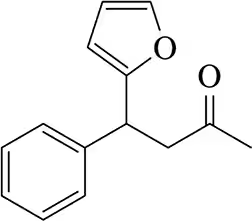
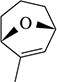
(a)
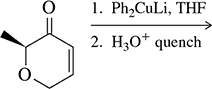
Problem 43b
Predict the product of the following reactions.
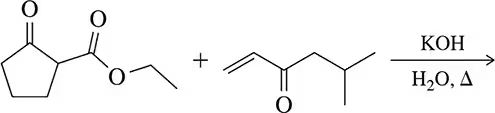
(b)
Problem 44
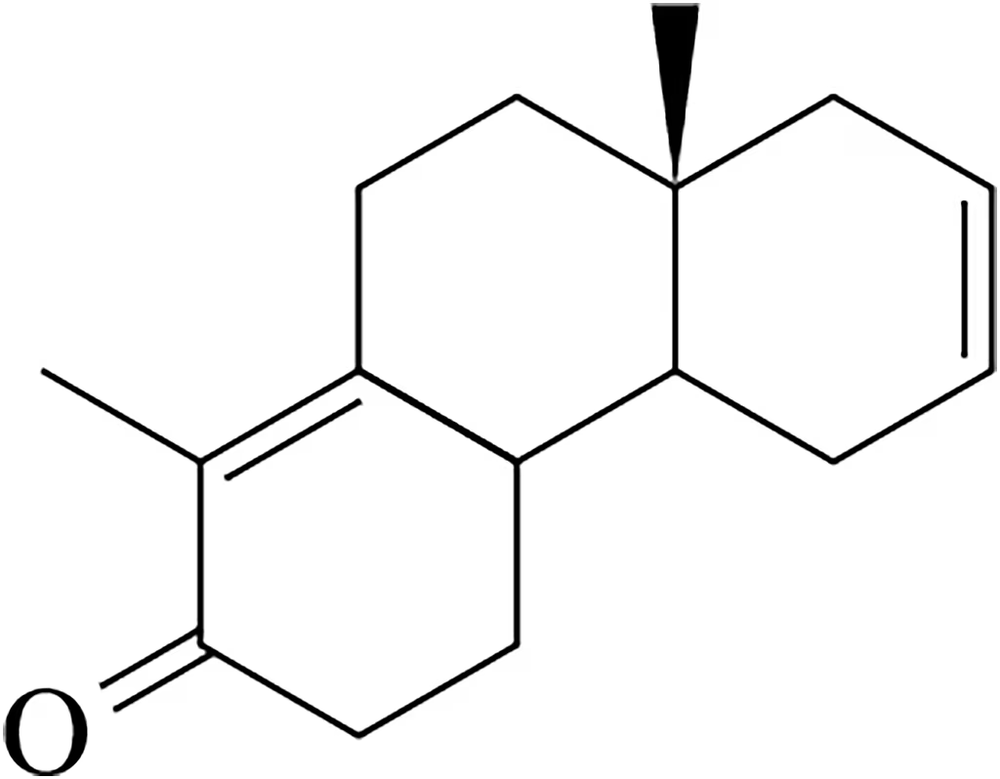
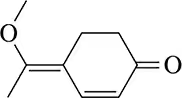
Show how the following molecule might be synthesized using the Robinson annulation.
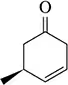
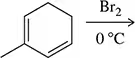
Problem 45a
Predict the product of the following reactions.
(a)
Problem 45b
Predict the product of the following reactions.
(b)
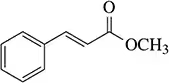
Problem 45c
Predict the product of the following reactions.
(c)
Problem 46a
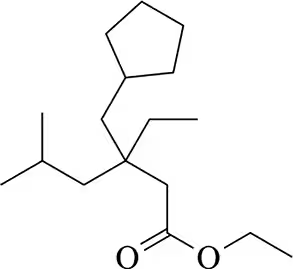
Suggest a reagent and a reactant that could be used to form the following molecules by conjugate addition of a cuprate. There can be multiple possibilities.
(a)
Problem 46b
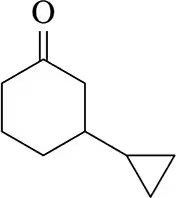
Suggest a reagent and a reactant that could be used to form the following molecules by conjugate addition of a cuprate. There can be multiple possibilities.
(b)
Problem 46c
Suggest a reagent and a reactant that could be used to form the following molecules by conjugate addition of a cuprate. There can be multiple possibilities.
(c)
Problem 47
To this point, we have seen four reactions that can be done by Gilman reagents. What are they? What do they have in common?
Problem 48
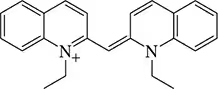
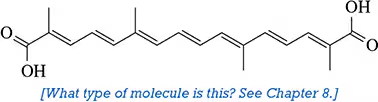
Would you expect the following molecules to absorb in the UV–visible region of the electromagnetic spectrum?
(a)
(b)
(c)
(d)
(e)
Problem 50
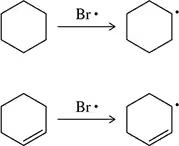
On a per alkene basis, which would have the more negative heat of hydrogenation?
Problem 51a
How many 𝝅 electrons are involved in the conjugated system for each of the following molecules?
(a)
Problem 53
Draw the molecular orbital picture of octa-1,3,5,7-tetraene, indicating which is the HOMO and which is the LUMO.
Problem 54b
Which of the following reactions would you expect to be faster/more favorable in each pair? Why?
(b)
Problem 54c
Which of the following reactions would you expect to be faster/more favorable in each pair? Why?
(c)
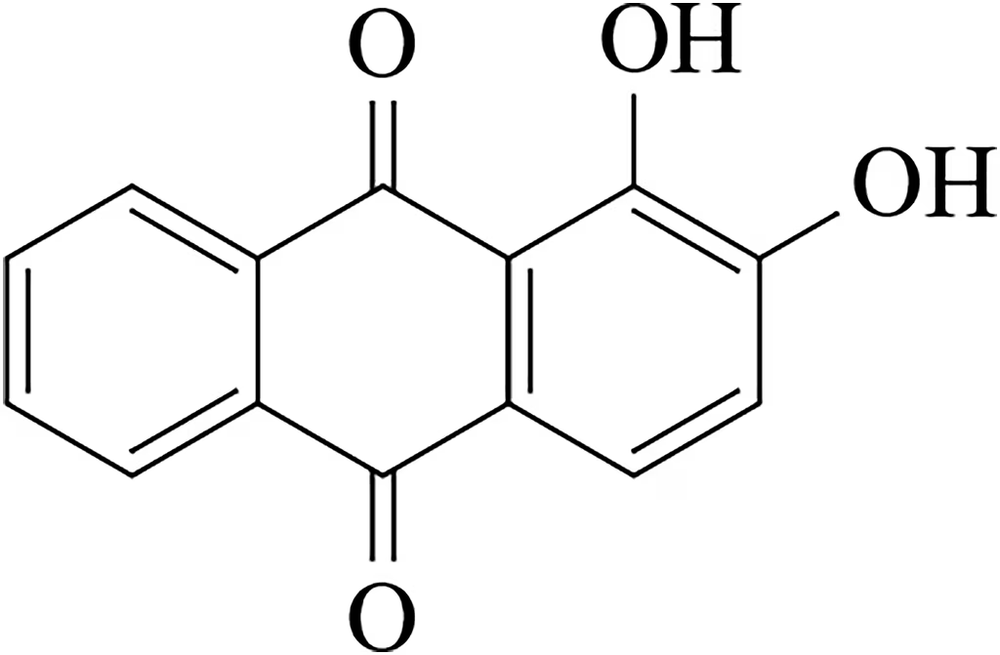
Problem 56
The dye alizarin normally forms an orange solution when dissolved. However, if KOEt is added to the solution, it turns blue very rapidly. Rationalize this result.
Problem 57d
Predict the product of the following reactions.
(d)
Problem 57i
Predict the product of the following reactions.
(i)
Problem 57j
Predict the product of the following reactions.
(j)
Problem 58
In Chapter 9, electron-rich alkenes were oxidized under acidic conditions with mCPBA. Conjugated alkenes can be oxidized using the same reagent, but under basic conditions. Suggest a mechanism for this reaction. [Think about what is electron-rich and what is electron-poor in the reaction. Also, identify the bonds formed and broken.]
Problem 60a
The reactivity of cyclopropanes often mimics that of alkenes.
(a) On the basis of this, suggest a mechanism for the following reaction.
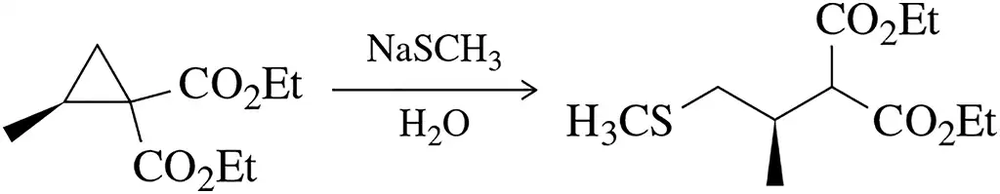
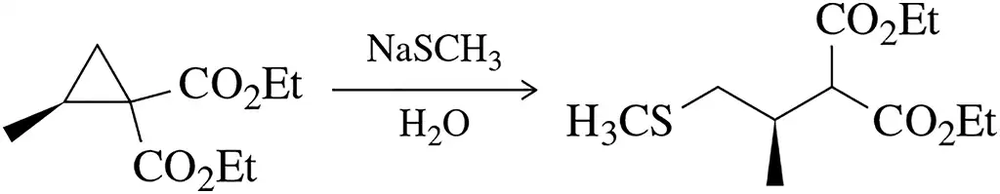
Problem 60b
The reactivity of cyclopropanes often mimics that of alkenes.
(b) Besides opening the three-membered ring, what is the driving force for this reaction?
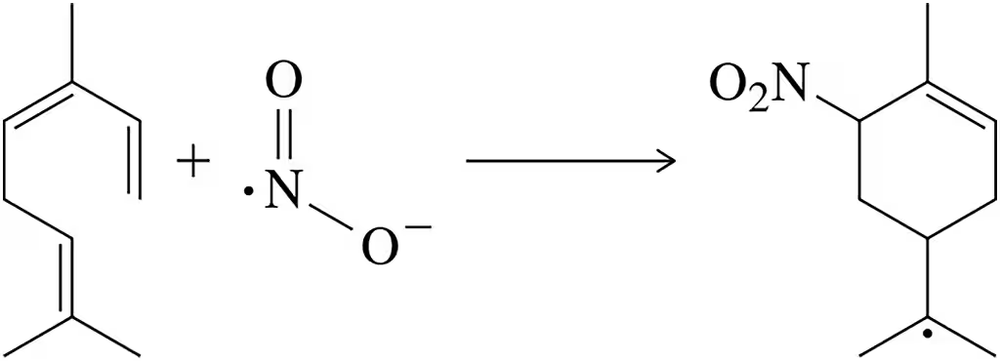
Problem 68
The following transformation was found to occur in areas with large NO₂ emissions. Suggest a mechanism for the reaction (J. Phys. Chem. 2013, 117, 14132–14140). [Hint: Use the fishhook arrows associated with radical reactions.]
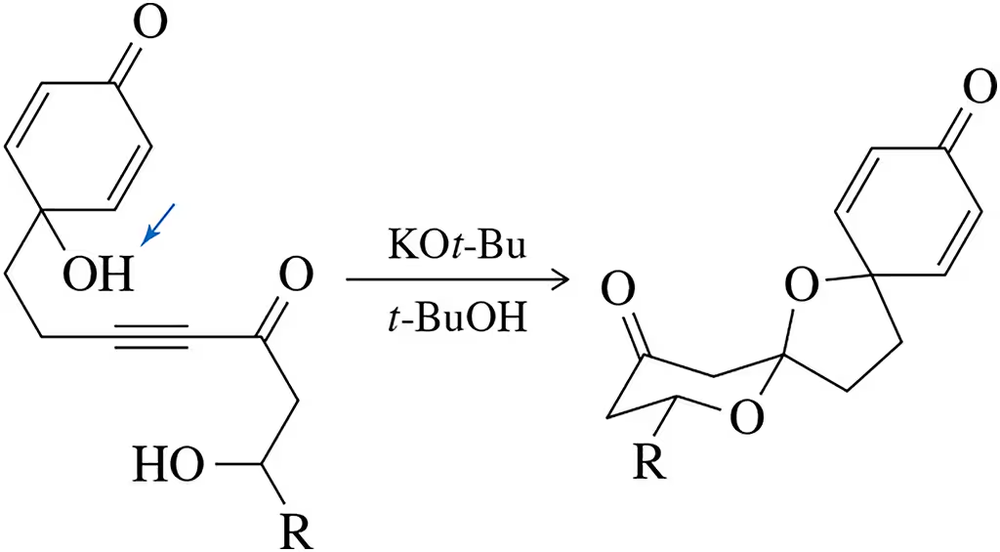
Problem 72
The following reaction was used in the synthesis of aculeatin A, a natural product that is active against KB cell lines. Although it only worked under acidic conditions, a mechanism can be drawn where the reaction might proceed under basic conditions. Suggest this mechanism (J. Org. Chem. 2014, 79, 1498–1504). [The most acidic proton is indicated . . . and number your carbons!]
Problem 73
The following covalent inhibitor blocks function in a protease found in the porcine epidemic diarrhea virus by reacting with a cysteine amino acid residue (shown below) in the active site. Draw the expected complex that forms between the inhibitor and the enzyme active site (J. Med. Chem. 2017, 60, 3212–3216.) [Assume the presence of active site bases if you need them.]
<IMAGE>