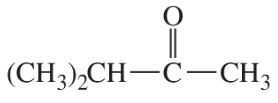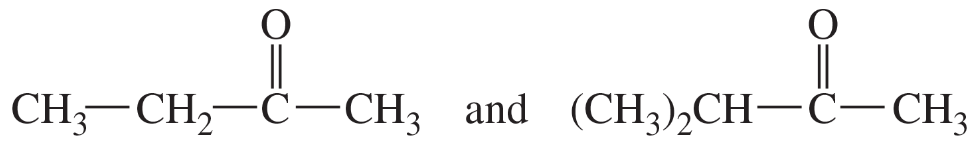Back
BackProblem 39a
Sketch your predictions of the proton NMR spectra of the following compounds.
(a) CH3–O–CH2CH3
Problem 39b
Sketch your predictions of the proton NMR spectra of the following compounds.
(b)
Problem 40a
Tell precisely how you would use the proton NMR spectra to distinguish between the following pairs of compounds.
(a) 1-bromopropane and 2-bromopropane
Problem 40b
Tell precisely how you would use the proton NMR spectra to distinguish between the following pairs of compounds.
(b)
Problem 43
A small pilot plant was adding bromine across the double bond of but-2-ene to make 2,3-dibromobutane. A controller malfunction allowed the reaction temperature to rise beyond safe limits. A careful distillation of the product showed that several impurities had formed, including the one having the NMR spectra that appear below. Determine its structure, and assign the peaks to the protons in your structure.
<IMAGE>
Problem 45
When 2-chloro-2-methylbutane is treated with a variety of strong bases, the products always seem to contain two isomers (A and B) of formula C5H10. When sodium hydroxide is used as the base, isomer A predominates. When potassium tert-butoxide is used as the base, isomer B predominates. The 1H and 13C NMR spectra of A and B are given below.
(a) Determine the structures of isomers A and B.
(b) Explain why A is the major product when using sodium hydroxide as the base and why B is the major product when using potassium tert-butoxide as the base.
<IMAGE>
Problem 46
(A true story.) A major university was designated as a national nuclear magnetic resonance center by the National Science Foundation. Several large superconducting instruments were being installed when a government safety inspector appeared and demanded to know what provisions were being made to handle the nuclear waste produced by these instruments. Assume you are the manager of the NMR center, and offer an explanation that could be understood by a nonscientist.
Problem 49
The three isomers of dimethylbenzene are commonly named ortho-xylene, meta-xylene, and para-xylene. These three isomers are difficult to distinguish using proton NMR, but they are instantly identifiable using 13C NMR.

(a) Describe how carbon NMR distinguishes these three isomers.
(b) Explain why they are difficult to distinguish using proton NMR.
Problem 50
(a) Draw all six isomers of formula C4H8 (including stereoisomers).
(b) For each structure, show how many types of H would appear in the proton NMR spectrum.
(c) For each structure, show how many types of C would appear in the 13C NMR spectrum.
(d) If an unknown compound of formula C4H8 shows two types of H and three types of C, can you determine its structure from this information?
Problem 51
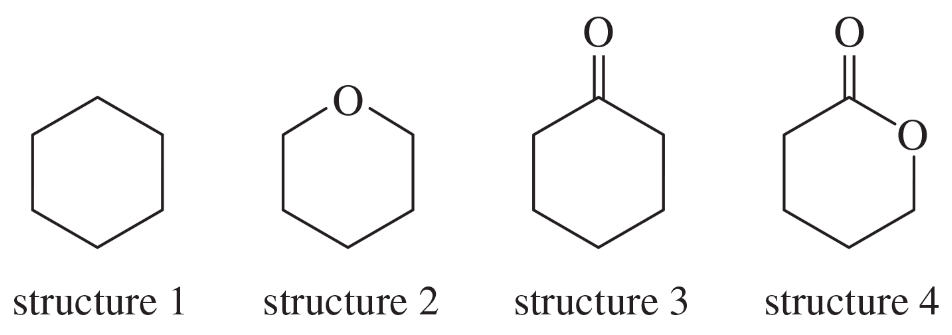
Different types of protons and carbons in alkanes tend to absorb at similar chemical shifts, making structure determination difficult. Explain how the 13C NMR spectrum, including the DEPT technique, would allow you to distinguish among the following four isomers.
Problem 52
Hexamethylbenzene undergoes free-radical chlorination to give one monochlorinated product (C12H17Cl) and four dichlorinated products (C12H16Cl2). These products are easily separated by GC-MS, but the dichlorinated products are difficult to distinguish by their mass spectra. Draw the monochlorinated product and the four dichlorinated products, and explain how 13C NMR would easily distinguish among these compounds.
Problem 53
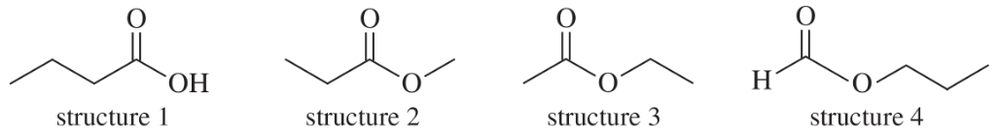
Each of these four structures has molecular formula C4H8O2. Match the structure with its characteristic proton NMR signals. (Not all of the signals are listed in each case.)
(a) sharp 1H singlet at δ8.0 and 2H triplet at δ4.0
(b) sharp 3H singlet at δ2.0 and 2H quartet at δ4.1
(c) sharp 3H singlet at δ3.7 and 2H quartet at δ2.3
(d) broad 1H singlet at δ11.5 and 2H triplet at δ2.3
Problem 54
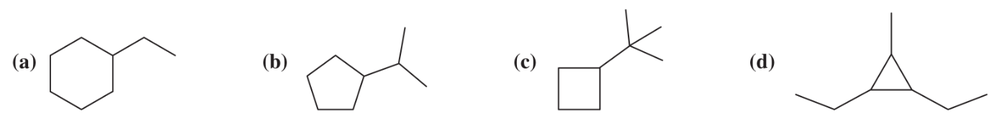
How many signals would you expect to see in the 13C NMR of the following compounds? In each case, show which carbon atoms are equivalent in the 13C NMR.
Problem 55
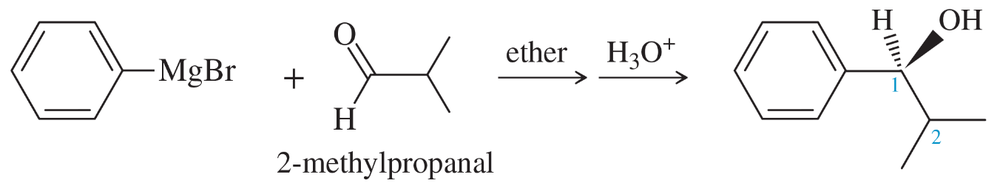
Phenyl Grignard reagent adds to 2-methylpropanal to give the secondary alcohol shown. The proton NMR of 2-methylpropanal shows the two methyl groups as equivalent (one doublet at δ1.1), yet the product alcohol, a racemic mixture, shows two different 3H doublets, one at δ0.75 and one around δ1.0.
(a) Draw a Newman projection of the product along the C1–C2 axis.
(b) Explain why the two methyl groups have different NMR chemical shifts. What is the term applied to protons such as these?
Problem 56
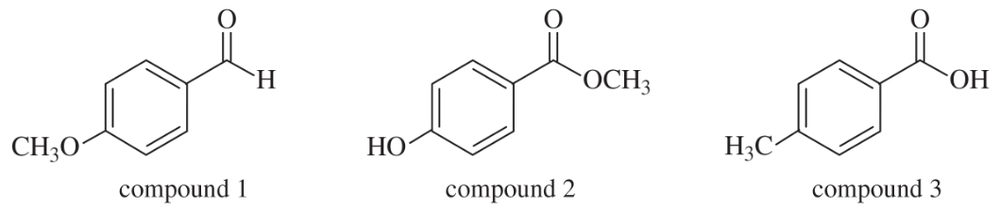
Show how you would distinguish among the following three compounds
(a) using infrared spectroscopy and no other information.
(b) using proton NMR spectroscopy and no other information.
(c) using 13C NMR, including DEPT, and no other information.