Back
BackProblem 74a
Show how the amino acid alanine can be synthesized from propanoic acid.
Problem 75b
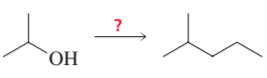
Show how the following compounds can be synthesized. The only carbon-containing compounds available to you for each synthesis are shown.
b.
Problem 76a,b,c
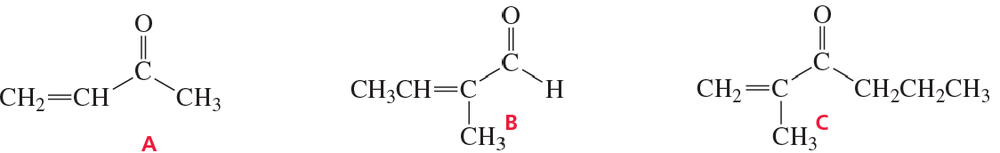
A student tried to prepare the following compounds using aldol condensations. Which of these compounds was she successful in synthesizing? Explain why the other syntheses were not successful.
Problem 78
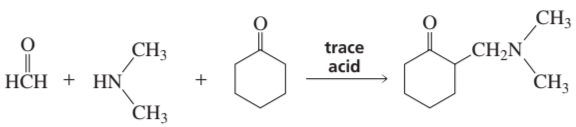
A Mannich reaction puts a
-group on the α-carbon of a ketone. Propose a mechanism for the reaction.
Problem 79
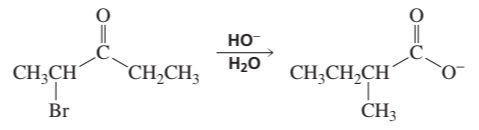
A carboxylic acid is formed when an a-haloketone reacts with hydroxide ion. This reaction is called a Favorskii reaction. Propose a mechanism for the following Favorskii reaction. (Hint: In the first step, HO- removes a proton from the a-carbon that is not bonded to Br; a three-membered ring is formed in the second step; and HO- is a nucleophile in the third step.)
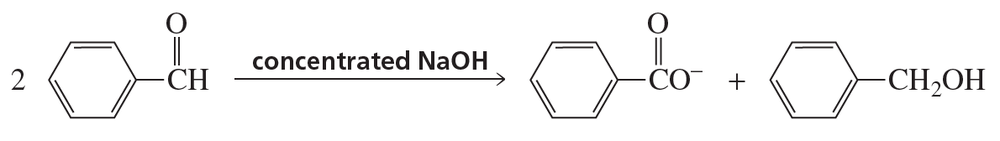
Problem 83
A Cannizzaro reaction is the reaction of an aldehyde that has no a-hydrogens with concentrated aqueous sodium hydroxide. In this reaction, half the aldehyde is converted to a carboxylic acid and the other half is converted to an alcohol. Propose a mechanism for the following Cannizzaro reaction:
Problem 84b
Propose a mechanism for each of the following reactions:
b.
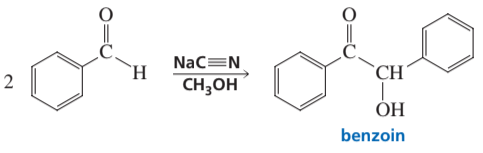
Problem 85
The following reaction is known as the benzoin condensation. The reaction does not take place if sodium hydroxide is used instead of sodium cyanide. Propose a mechanism for the reaction and explain why the reaction does not occur if hydroxide ion is the base.
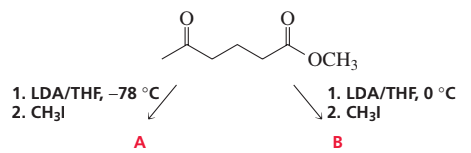
Problem 87a
Alkylation of the following compound with methyl iodide under two different conditions forms two different ketoesters (A and B). Each ketoester forms a cyclic diketone (C and D) when treated with methoxide ion in methanol. a. Draw the structures of A and B, and indicate the conditions used in the alkylation reaction that cause that ketoester to be formed.
Problem 89b
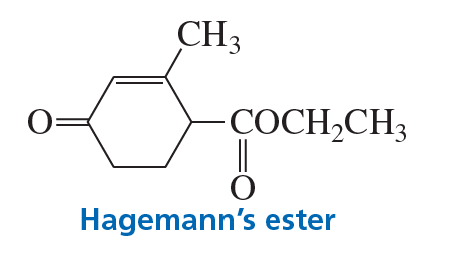
A compound known as Hagemann's ester can be prepared by treating a mixture of formaldehyde and ethyl acetoacetate first with base and then with acid and heat. Write the structure for the product of each of the steps.
b. The second step is a Michael addition.
Problem 90
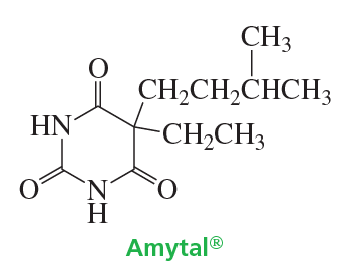
Amobarbital is a sedative marketed under the trade name Amytal. Propose a synthesis of amobarbital, using diethyl malonate and urea as two of the starting materials.