Textbook Question
How would the lock-and-key model explain that sucrase hydrolyzes sucrose, but not lactose?

 Verified step by step guidance
Verified step by step guidance

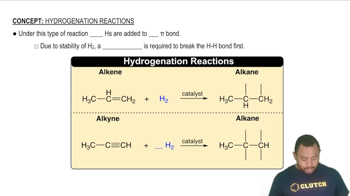
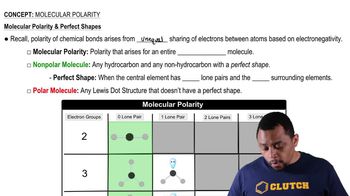
How would the lock-and-key model explain that sucrase hydrolyzes sucrose, but not lactose?
If a blood test indicates a high level of ALT, what could be the cause?
Consider the amino acids lysine, valine, and aspartate in an enzyme. State which of these amino acids have R groups that would:
b. be found in hydrophilic regions
Consider the amino acids lysine, valine, and aspartate in an enzyme. State which of these amino acids have R groups that would:
d. form salt bridges
Consider the amino acids histidine, phenylalanine, and serine in an enzyme. State which of these amino acids have R groups that would:
d. form salt bridges