Back
BackProblem 17c
Evaluate each of the following:
c. 4 × (–2) + 6 = __________
Problem 22d
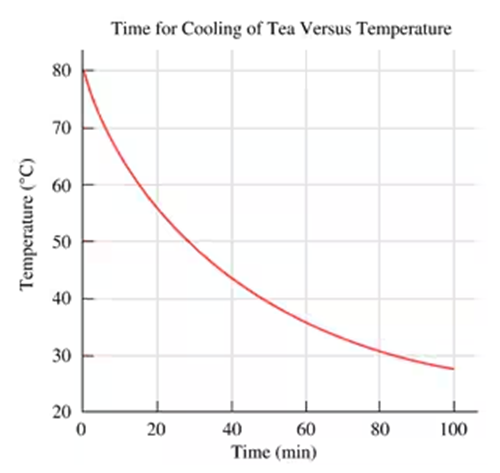
Use the following graph for problems 1.23 and 1.24:
How many minutes were needed to reach a temperature of 45 °C?
Problem 24b
An alloy contains 67 g of pure gold and 35 g of pure zinc. What is the percentage of zinc in the alloy? Express your answer to the ones place.
Problem 30a
Write each of the following in scientific notation:
a. 0.024
Problem 30b
Write each of the following in scientific notation:
b. 1500
Problem 30c
Write each of the following in scientific notation:
c. 0.000 62
Problem 30d
Write each of the following in scientific notation:
d. 360 000
Problem 33
A container was found in Gloria's home that contains 140 g of ethylene glycol in 480 g of liquid. What is the percentage of ethylene glycol? Express your answer to the ones place.
Problem 34
If the toxic quantity is 1.5 g of ethylene glycol per 1000 g of body mass, what percentage of ethylene glycol is fatal?
Problem 51a
A bag of gumdrops contains 16 orange gumdrops, 8 yellow gumdrops, and 16 black gumdrops.
a. What is the percentage of yellow gumdrops? Express your answer to the ones place.
Problem 55a
Write each of the following in scientific notation:
a. 0.000 026
Problem 55b
Write each of the following in scientific notation:
b. 650
Problem 55c
Write each of the following in scientific notation:
c. 0.37
Problem 55d
Write each of the following in scientific notation:
d. 530 000
Problem 56a
Write each of the following in scientific notation:
a. 0.072
Problem 56b
Write each of the following in scientific notation:
b. 1440
Problem 56c
Write each of the following in scientific notation:
c. 0.000 48
Problem 56d
Write each of the following in scientific notation:
d. 9 100 000
Problem 63d
Use the following graph for problems 1.55 and 1.56:
<IMAGE>
d. Does the solubility of carbon dioxide increase or decrease with an increase in temperature?