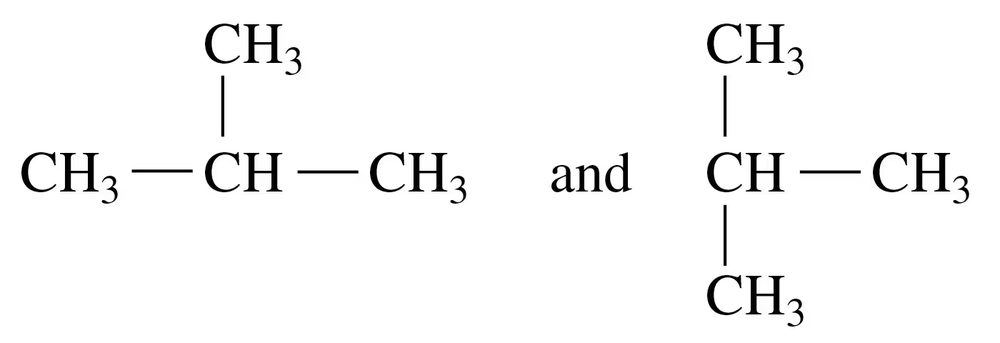Back
BackProblem 1b
Identify each of the following as a formula of an organic or inorganic compound. For an organic compound, indicate if represented as molecular formula, expanded, or condensed structural formula:
b. CH3―CH2―CH2―CH3
Problem 1c
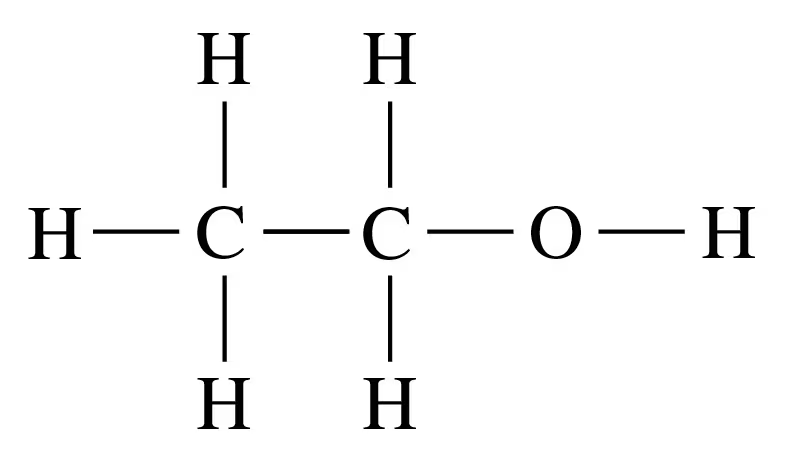
Identify each of the following as a formula of an organic or inorganic compound. For an organic compound, indicate if represented as molecular formula, expanded, or condensed structural formula:
c.
Problem 1f
Identify each of the following as a formula of an organic or inorganic compound. For an organic compound, indicate if represented as molecular formula, expanded, or condensed structural formula:
f. C3H7Cl
Problem 2a
Identify each of the following as a formula of an organic or inorganic compound. For an organic compound, indicate if represented as molecular formula, expanded, or condensed structural formula:
a. C6H12O6
Problem 2b
Identify each of the following as a formula of an organic or inorganic compound. For an organic compound, indicate if represented as molecular formula, expanded, or condensed structural formula:
b. K3PO4
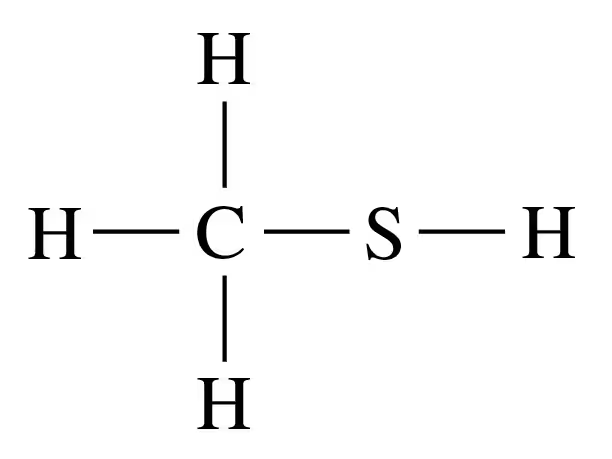
Problem 2d
Identify each of the following as a formula of an organic or inorganic compound. For an organic compound, indicate if represented as molecular formula, expanded, or condensed structural formula:
d.
Problem 2e
Identify each of the following as a formula of an organic or inorganic compound. For an organic compound, indicate if represented as molecular formula, expanded, or condensed structural formula:
e. CH3―CH2―CH2―CH2―CH3
Problem 3b
Identify each of the following properties as more typical of an organic or inorganic compound:
a. is soluble in water
Problem 3c
Identify each of the following properties as more typical of an organic or inorganic compound:
c. contains carbon and hydrogen
Problem 4a
Identify each of the following properties as more typical of an organic or inorganic compound:
b. is a gas at room temperature
Problem 4c
Identify each of the following properties as more typical of an organic or inorganic compound:
c. contains covalent bonds
Problem 5b
Match each of the following physical and chemical properties with ethane, C2H6 or sodium bromide, NaBr:
b. burns vigorously in air
Problem 5c
Match each of the following physical and chemical properties with ethane, C2H6 or sodium bromide, NaBr:
a. boils at -89 °C
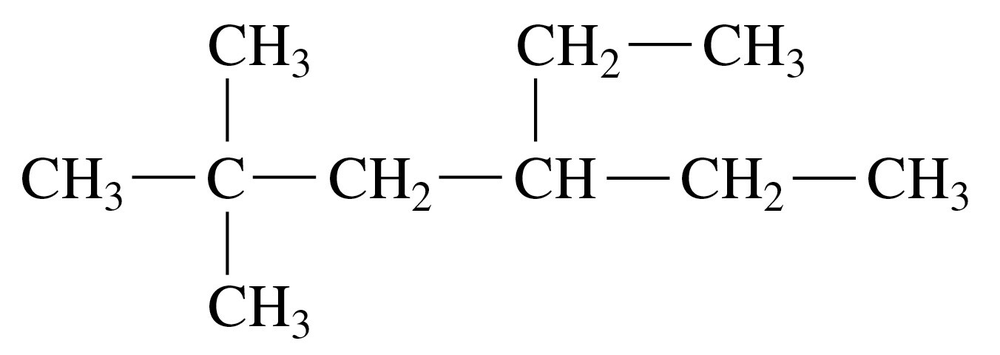
Problem 7a
Give the IUPAC name for each of the following alkanes and cycloalkanes:
a. <IMAGE>
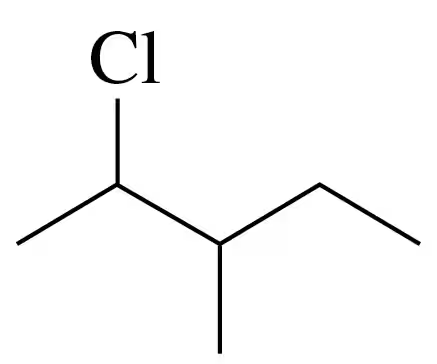
Problem 7c
Give the IUPAC name for each of the following alkanes and cycloalkanes:
c.
Problem 10a
Draw the condensed structural formula for alkanes or the line-angle formula for cycloalkanes for each of the following:
c. heptane
Problem 11a
Indicate whether each of the following pairs represent structural isomers or the same molecule:
a.
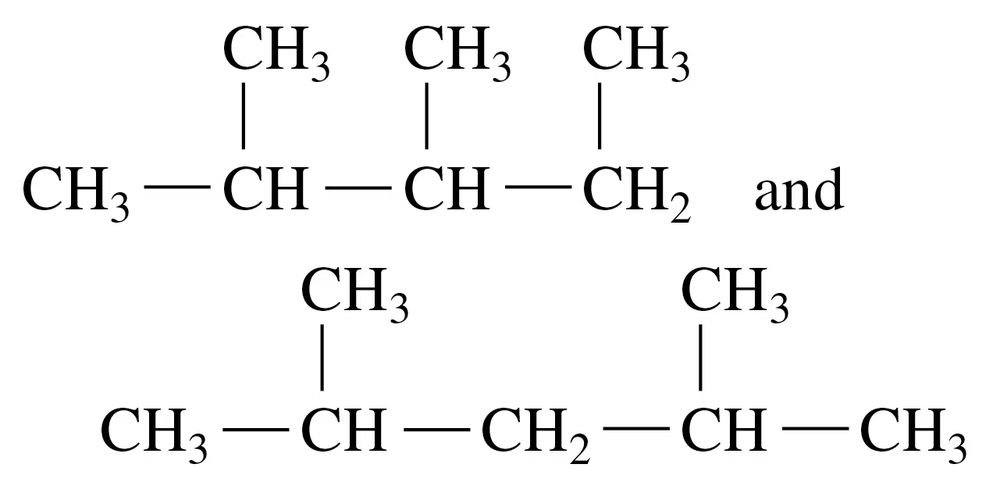
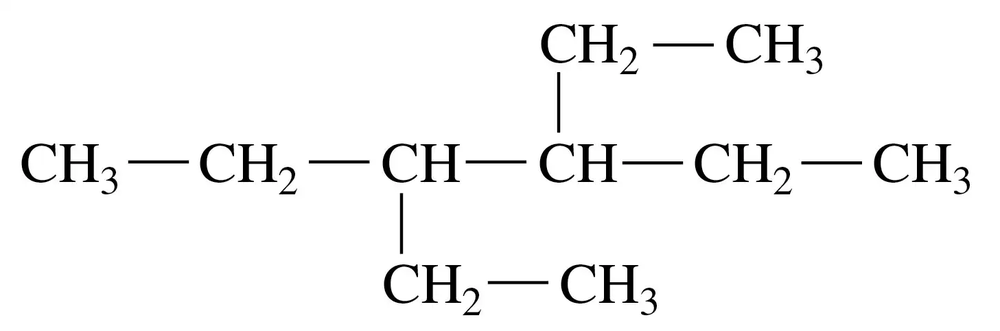
Problem 12b
Indicate whether each of the following pairs represent structural isomers or the same molecule:
b.
Problem 13b
Give the IUPAC name for each of the following:
c.
Problem 13c
Give the IUPAC name for each of the following:
b.
Problem 14a
Give the IUPAC name for each of the following:
c.
Problem 15d
Draw the condensed structural formula for each of the following alkanes:
d. 1-bromo-2-chloroethane
Problem 17a
Draw the line-angle formula for each of the following:
a. 3-methylheptane
Problem 17b
Draw the line-angle formula for each of the following:
b. ethylcyclopentane
Problem 17d
Draw the line-angle formula for each of the following:
d. 2,3-dichlorohexane
Problem 19d
Heptane, used as a solvent for rubber cement, has a density of 0.68 g/mL, the melting point is -91 °C, and the boiling point 98 °C.
d. Will heptane float on water or sink?
Problem 20c
Nonane has a density of 0.79 g/mL, the melting point is -53 °C, and the boiling point 151 °C.
c. Is nonane soluble in water?
Problem 21b
Write the balanced chemical equation for the complete combustion of each of the following compounds:
b. cyclopropane
Problem 21c
Write the balanced chemical equation for the complete combustion of each of the following compounds:
c. 2,3-dimethylhexane
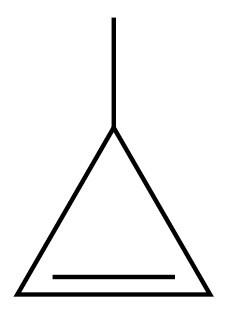
Problem 24a
Identify the following as alkanes, alkenes, cycloalkenes, or alkynes:
a.