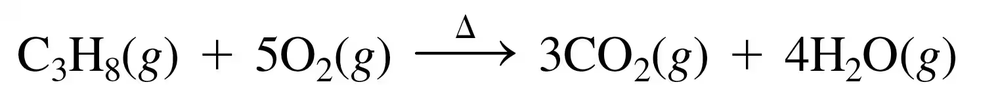Back
BackProblem 1
What is a mole?
Problem 2
What is Avogadro's number?
Problem 11b
Calculate the molar mass for each of the following:
b. C3H6O3
Problem 12b
Calculate the molar mass for each of the following:
c. Fe(ClO4)3
Problem 17a
Calculate the molar mass for each of the following:
a. Al2(SO4)3, antiperspirant
Problem 22d
Calculate the mass, in grams, for each of the following:
d. 0.145 mole of C2H6O
Problem 22e
Calculate the mass, in grams, for each of the following:
e. 2.08 moles of (NH4)2SO4
Problem 35d
Determine whether each of the following chemical equations is balanced or not balanced:
d.
Problem 38b
Balance each of the following chemical equations:
c. Sb2S3(s) + HCl(aq) → SbCl3(aq) + H2S(g)
Problem 39d
Balance each of the following chemical equations:
d. Al(s) + HCl(aq) → H2(g) + AlCl3(aq)
Problem 45c
Identify each of the following as an oxidation or a reduction:
c. Cr3+(aq) + 3e– → Cr(s)
Problem 49a
In the mitochondria of human cells, energy is provided by the oxidation and reduction reactions of the iron ions in the cytochromes in electron transport. Identify each of the following as an oxidation or a reduction:
a. Fe3+ + e– → Fe2+
Problem 55c
The chemical reaction of hydrogen with oxygen produces water.
2 H2(g) + O2(g) → 2 H2O(g)
c. How many moles of H2O form when 2.5 moles of O2 reacts?
Problem 67a
Why do chemical reactions require energy of activation?
Problem 67c
Draw an energy diagram for an exothermic reaction.
Problem 68a
What is measured by the heat of reaction?
Problem 69b
Classify each of the following as exothermic or endothermic:
b. The energy level of the products is higher than that of the reactants.
Problem 69c
Classify each of the following as exothermic or endothermic:
c. The metabolism of glucose in the body provides energy.
Problem 70a
Classify each of the following as exothermic or endothermic:
b. In the body, the synthesis of proteins requires energy.
Problem 73a
What is meant by the rate of a reaction?
Problem 75a
How would each of the following change the rate of the reaction shown here? 2SO2(g) + O2(g) → 2SO3(g)
a. adding more SO2(g)
Problem 76c
How would each of the following change the rate of the reaction shown here?
2 NO(g) + 2 H2(g) → N2(g) + 2 H2O(g)
c. removing some H2(g)
Problem 79c
Using the models of the molecules (black = C, white = H, yellow = S, green = Cl), determine each of the following for models of compounds 1 and 2:
d. number of moles in 10.0 g
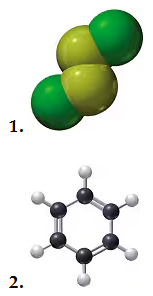
Problem 80c
Using the models of the molecules (black = C, white = H, yellow = S, red = O), determine each of the following for models of compounds 1 and 2:
c. number of moles in 10.0 g
Problem 83
Balance each of the following by adding coefficients, and identify the type of reaction for each:
a.
Problem 85a
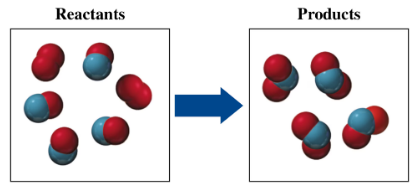
If red spheres represent oxygen atoms, blue spheres represent nitrogen atoms, and all the molecules are gases,
a. write the formula for each of the reactants and products.
Problem 85b
If red spheres represent oxygen atoms, blue spheres represent nitrogen atoms, and all the molecules are gases,
b. write a balanced equation for the reaction.
Problem 85c
If red spheres represent oxygen atoms, blue spheres represent nitrogen atoms, and all the molecules are gases,
c. indicate the type of reaction as combination, decomposition, single replacement, double replacement, or combustion.
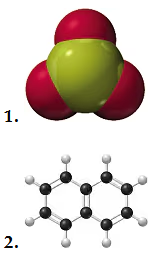
Problem 86a
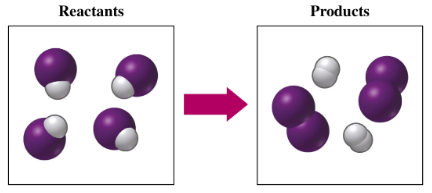
If purple spheres represent iodine atoms, white spheres represent hydrogen atoms, and all the molecules are gases,
a. write the formula for each of the reactants and products.
Problem 86b
If purple spheres represent iodine atoms, white spheres represent hydrogen atoms, and all the molecules are gases,
b. write a balanced equation for the reaction.