Oxaloacetate is an inhibitor of succinate dehydrogenase.
a. Would you expect oxaloacetate to be a competitive or a noncompetitive inhibitor? Why?

 Verified step by step guidance
Verified step by step guidance
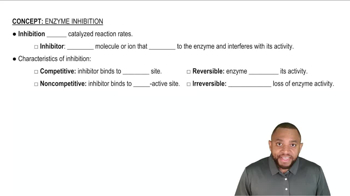


Oxaloacetate is an inhibitor of succinate dehydrogenase.
a. Would you expect oxaloacetate to be a competitive or a noncompetitive inhibitor? Why?
Methanol and ethanol are oxidized by alcohol dehydrogenase. In methanol poisoning, ethanol is given intravenously to prevent the formation of formaldehyde that has toxic effects.
b. Would ethanol compete for the active site or bind to a different site?
In humans, the antibiotic amoxicillin (a type of penicillin) is used to treat certain bacterial infections.
a. Does the antibiotic inhibit enzymes in humans?
Ethylene glycol (HO—CH2—CH2—OH) is a major component of antifreeze. If ingested, it is first converted to HOOC—CHO (oxoethanoic acid) and then to HOOC—COOH (oxalic acid), which is toxic.
<IMAGE>
a. What class of enzyme catalyzes the reactions described?
Ethylene glycol (HO—CH2—CH2—OH) is a major component of antifreeze. If ingested, it is first converted to HOOC—CHO (oxoethanoic acid) and then to HOOC—COOH (oxalic acid), which is toxic.
<IMAGE>
b. The treatment for the ingestion of ethylene glycol is an intravenous solution of ethanol. How might this help prevent toxic levels of oxalic acid in the body?
Adults who are lactose intolerant cannot break down the disaccharide in milk products. To help digest dairy food, a product known as Lactaid can be given prior to consuming dairy products. (16.4, 16.5)
a. What is the name of enzyme present in Lactaid, and what is the major class of this enzyme?