In humans, the antibiotic amoxicillin (a type of penicillin) is used to treat certain bacterial infections.
a. Does the antibiotic inhibit enzymes in humans?

 Verified step by step guidance
Verified step by step guidance
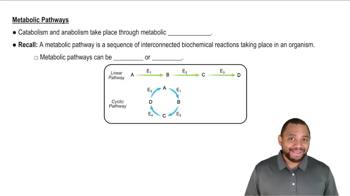

In humans, the antibiotic amoxicillin (a type of penicillin) is used to treat certain bacterial infections.
a. Does the antibiotic inhibit enzymes in humans?
In humans, the antibiotic amoxicillin (a type of penicillin) is used to treat certain bacterial infections.
c. Is amoxicillin a reversible or irreversible inhibitor?
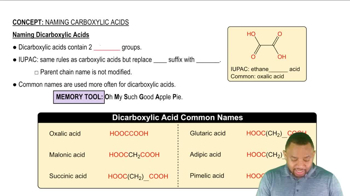
Ethylene glycol (HO—CH2—CH2—OH) is a major component of antifreeze. If ingested, it is first converted to HOOC—CHO (oxoethanoic acid) and then to HOOC—COOH (oxalic acid), which is toxic.
<IMAGE>
a. What class of enzyme catalyzes the reactions described?
Adults who are lactose intolerant cannot break down the disaccharide in milk products. To help digest dairy food, a product known as Lactaid can be given prior to consuming dairy products. (16.4, 16.5)
a. What is the name of enzyme present in Lactaid, and what is the major class of this enzyme?
Adults who are lactose intolerant cannot break down the disaccharide in milk products. To help digest dairy food, a product known as Lactaid can be given prior to consuming dairy products. (16.4, 16.5)
b. What might happen to the enzyme if the Lactaid were stored at 55 °C?
Fresh pineapple contains the enzyme bromelain that hydrolyzes peptide bonds in proteins.
<IMAGE>
a. The directions for a gelatin (protein) dessert say not to add fresh pineapple. However, canned pineapple where pineapple is heated to high temperatures can be added. Why?