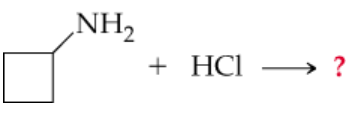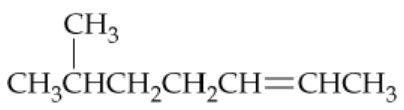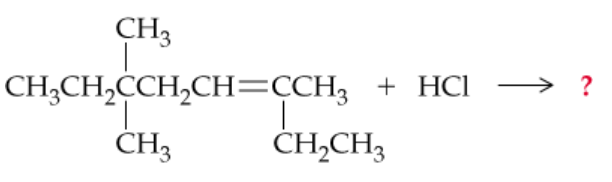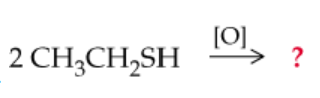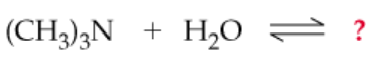Back
BackProblem 42a
Complete the following equations. (Hint: Remember that a nitrogen with three groups bound to it has a lone pair and one with four does not)
a.
Problem 43
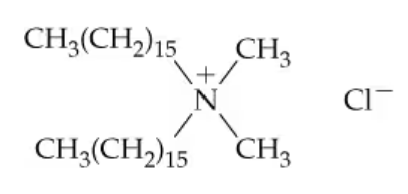
Many hair conditioners contain an ammonium salt such as the following to help prevent 'fly-away' hair. These ions will react with neither acid nor base. Provide a reason why.
Problem 44
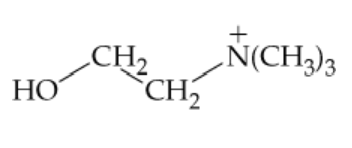
Choline has the following structure. Do you think that this substance reacts with aqueous hydrochloric acid? If so, what is the product? If not, why not?
Problem 49
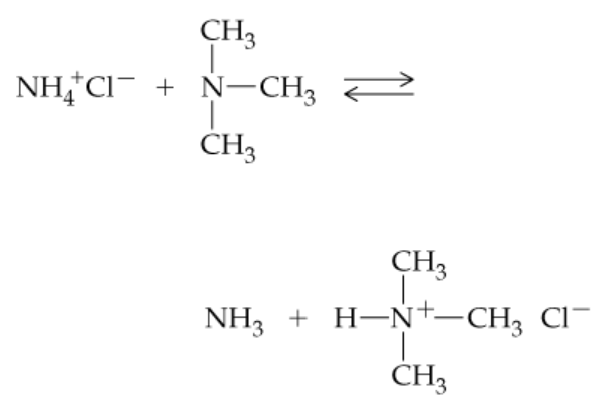
Which is the stronger base, trimethylamine or ammonia? In which direction will the following reaction proceed?
Problem 50
How do amines differ from analogous alcohols in (a) odor, (b) basicity, and (c) boiling point?
Problem 52a
Name the following compounds:
a.
Problem 53a
Complete the following equations (Hint: Answers may include concepts learned from previous organic chapters):
a.
Problem 53c
Complete the following equations (Hint: Answers may include concepts learned from previous organic chapters):
c.
Problem 53e
Complete the following equations (Hint: Answers may include concepts learned from previous organic chapters):
e.
Problem 55
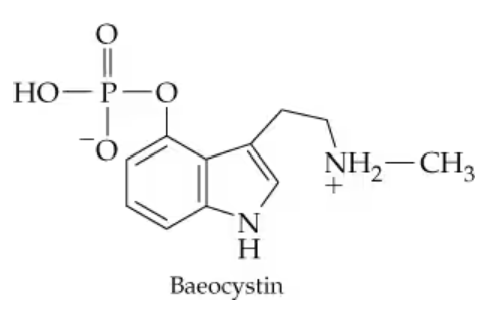
Baeocystin is a hallucinogenic compound that is isolated from the mushroom Psilocybe baeocystis and has the structure shown below. What heterocyclic base (Table 16.1) is the parent of this compound?
Problem 57
Benzene and pyridine are both single-ring, aromatic compounds. Benzene is a neutral compound that is insoluble in water. Pyridine, with a similar molar mass, is basic and completely miscible with water. Explain these phenomena.
Problem 61
Lemon juice, which contains citric acid, is traditionally recommended for removing the odor associated with cleaning fish. What functional group is responsible for a 'fishy' odor, and why does lemon juice work to remove the odor? If possible, test this at home using a piece of fish.