Germanium has the same structure as silicon, but the unit cell size is different because Ge and Si atoms are not the same size. If you were to repeat the experiment described in Additional Exercise 12.117, but replace the Si crystal with a Ge crystal, would you expect the X rays to be diffracted at a larger or smaller angle 𝜃?
Ch.12 - Solids and Modern Materials

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 12, Problem 120
When you shine light of band gap energy or higher on a semiconductor and promote electrons from the valence band to the conduction band, do you expect the conductivity of the semiconductor to (a) remain unchanged, (b) increase, or (c) decrease?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the structure of a semiconductor. Semiconductors have a valence band filled with electrons and a conduction band that is typically empty. The energy gap between these two bands is known as the band gap.
Step 2: Consider what happens when light with energy equal to or greater than the band gap is shone on the semiconductor. This energy can excite electrons from the valence band to the conduction band.
Step 3: Recognize that when electrons are promoted to the conduction band, they leave behind holes in the valence band. Both the electrons in the conduction band and the holes in the valence band can contribute to electrical conductivity.
Step 4: Analyze the effect of increased charge carriers. With more electrons in the conduction band and more holes in the valence band, the number of charge carriers increases, which typically enhances the material's ability to conduct electricity.
Step 5: Conclude that the conductivity of the semiconductor is expected to increase when light of band gap energy or higher is shone on it, due to the increased number of charge carriers.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Band Gap Energy
Band gap energy is the energy difference between the valence band, where electrons are normally present, and the conduction band, where electrons can move freely and contribute to electrical conductivity. In semiconductors, this gap is small enough that when energy, such as light, is applied, electrons can be excited from the valence band to the conduction band, allowing for increased conductivity.
Recommended video:
Guided course
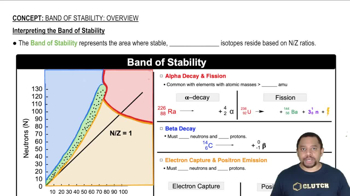
Intepreting the Band of Stability
Electron Promotion
Electron promotion refers to the process of moving electrons from a lower energy state (valence band) to a higher energy state (conduction band) when sufficient energy is provided. This transition is crucial in semiconductors, as it directly affects their ability to conduct electricity. The promotion of electrons increases the number of charge carriers available for conduction, thereby enhancing the material's conductivity.
Recommended video:
Guided course

Electron Configuration Exceptions Example
Conductivity in Semiconductors
Conductivity in semiconductors is a measure of how easily electric current can flow through the material. It is influenced by the number of charge carriers (electrons and holes) present. When light of sufficient energy is shone on a semiconductor, it promotes electrons to the conduction band, increasing the number of free charge carriers and thus raising the conductivity of the semiconductor.
Recommended video:
Guided course
Extensive Property Example
Related Practice
Textbook Question
Textbook Question
(a) The density of diamond is 3.5 g/cm3, and that of graphite is 2.3 g/cm3. Based on the structure of buckminsterfullerene, what would you expect its density to be relative to these other forms of carbon?
Textbook Question
(b) X-ray diffraction studies of buckminsterfullerene show that it has a face-centered cubic lattice of C60 molecules. The length of an edge of the unit cell is 14.2 Å. Calculate the density of buckminsterfullerene.
Textbook Question
(a) What are the C—C—C bond angles in diamond?
1
views
Textbook Question
(c) What atomic orbitals are involved in the stacking of graphite sheets with each other?
