(a) The density of diamond is 3.5 g/cm3, and that of graphite is 2.3 g/cm3. Based on the structure of buckminsterfullerene, what would you expect its density to be relative to these other forms of carbon?
Ch.12 - Solids and Modern Materials

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 12, Problem 121
Spinel is a mineral that contains 37.9% Al, 17.1% Mg, and 45.0% O, by mass, and has a density of 3.57 g/cm³. The unit cell is cubic with an edge length of 8.09 Å. How many atoms of each type are in the unit cell?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Calculate the molar mass of the compound using the given percentages and the atomic masses of Al, Mg, and O. Assume 100 g of the compound to simplify calculations.
Step 2: Convert the mass percentages to moles by dividing the mass of each element by its respective atomic mass (Al: 26.98 g/mol, Mg: 24.31 g/mol, O: 16.00 g/mol).
Step 3: Determine the mole ratio of Al, Mg, and O by dividing the number of moles of each element by the smallest number of moles calculated in Step 2.
Step 4: Use the density and the volume of the unit cell to calculate the mass of the unit cell. The volume of the unit cell is calculated using the edge length: \( V = (8.09 \times 10^{-8} \text{ cm})^3 \).
Step 5: Calculate the number of formula units per unit cell by dividing the mass of the unit cell by the molar mass of the compound. Use this to determine the number of atoms of each type in the unit cell based on the mole ratio.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Mass and Composition
To determine the number of atoms in the unit cell, one must first calculate the molar mass of each element present in the mineral. The percentages by mass of aluminum (Al), magnesium (Mg), and oxygen (O) can be used to find the empirical formula, which indicates the ratio of atoms in the compound. This is essential for converting mass percentages into moles, which will ultimately help in finding the number of atoms in the unit cell.
Recommended video:
Guided course

Molar Mass Concept
Unit Cell and Atomic Arrangement
The unit cell is the smallest repeating unit in a crystal lattice that reflects the symmetry and structure of the entire crystal. In a cubic unit cell, the arrangement of atoms can vary, affecting how many of each type of atom are present. Understanding the geometry and the edge length of the unit cell is crucial for calculating the total number of atoms based on the volume and density of the mineral.
Recommended video:
Guided course
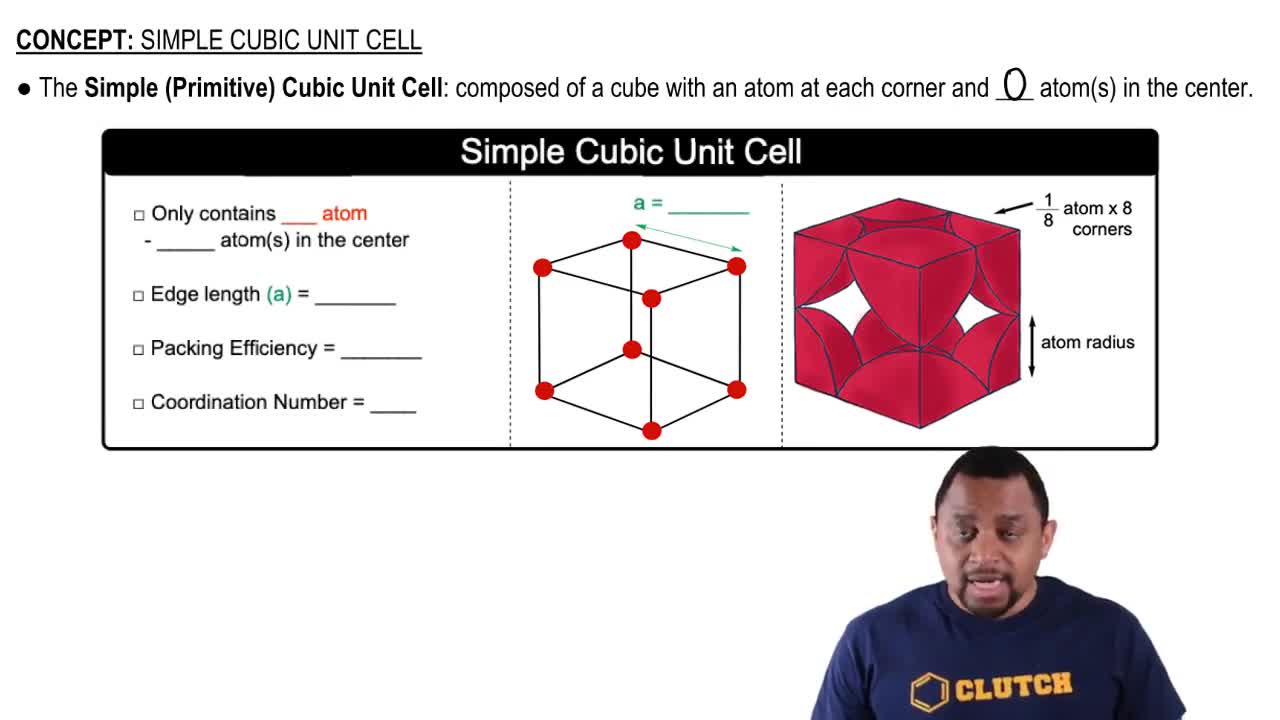
Simple Cubic Unit Cell
Density and Volume Calculations
Density is defined as mass per unit volume and is a key factor in determining the number of atoms in a unit cell. By using the given density of spinel and the calculated volume from the edge length of the cubic unit cell, one can find the total mass of the unit cell. This mass can then be related back to the number of moles and subsequently the number of atoms of each element present in the unit cell.
Recommended video:
Guided course
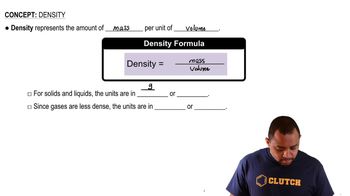
Density Concepts
Related Practice
Textbook Question
Textbook Question
(b) X-ray diffraction studies of buckminsterfullerene show that it has a face-centered cubic lattice of C60 molecules. The length of an edge of the unit cell is 14.2 Å. Calculate the density of buckminsterfullerene.
Textbook Question
(a) What are the C—C—C bond angles in diamond?
1
views
Textbook Question
(c) What atomic orbitals are involved in the stacking of graphite sheets with each other?
Textbook Question
Employing the bond enthalpy values listed in Table 8.4, estimate the molar enthalpy change occurring upon (a) polymerization of ethylene. (b) formation of nylon 6,6. (c) formation of polyethylene terephthalate (PET).
