In their study of X-ray diffraction, William and Lawrence Bragg determined that the relationship among the wavelength of the radiation 1l2, the angle at which the radiation is diffracted 1u2, and the distance between planes of atoms in the crystal that cause the diffraction (d) is given by nl = 2d sin u. X rays from a copper X-ray tube that have a wavelength of 1.54 Å are diffracted at an angle of 14.22 degrees by crystalline silicon. Using the Bragg equation, calculate the distance between the planes of atoms responsible for diffraction in this crystal, assuming n = 1 (first-order diffraction).
Ch.12 - Solids and Modern Materials

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 12, Problem 119a
(a) The density of diamond is 3.5 g/cm3, and that of graphite is 2.3 g/cm3. Based on the structure of buckminsterfullerene, what would you expect its density to be relative to these other forms of carbon?
 Verified step by step guidance
Verified step by step guidance1
Understand the structures: Diamond has a 3D tetrahedral structure, graphite has a layered planar structure, and buckminsterfullerene (C60) has a spherical structure.
Consider the packing efficiency: Diamond's structure is very dense due to its strong covalent bonds in a 3D network, while graphite's layers are less densely packed due to weaker van der Waals forces between layers.
Buckminsterfullerene's structure is a hollow sphere, which suggests it might have a lower density than diamond due to less efficient packing, but potentially similar or slightly higher than graphite due to its closed structure.
Compare the densities: Since diamond is the densest due to its structure and graphite is less dense, buckminsterfullerene's density is expected to be between that of graphite and diamond.
Conclude the relative density: Based on the structural considerations, buckminsterfullerene is likely to have a density greater than graphite but less than diamond.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Density
Density is defined as mass per unit volume, typically expressed in grams per cubic centimeter (g/cm³). It is a physical property that can help distinguish between different materials. In the context of carbon allotropes, density can provide insights into their structural arrangement and bonding, influencing how tightly the atoms are packed.
Recommended video:
Guided course
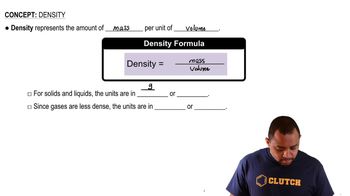
Density Concepts
Allotropes of Carbon
Carbon exhibits several allotropes, including diamond, graphite, and buckminsterfullerene, each with distinct structural properties. Diamond has a tetrahedral lattice structure, leading to high density, while graphite has a layered structure that allows for lower density. Buckminsterfullerene, with its spherical structure, is expected to have a density that falls between those of diamond and graphite due to its unique arrangement of carbon atoms.
Recommended video:
Guided course
3 and 4 Carbon Alkyls
Molecular Structure and Density Relationship
The molecular structure of a substance significantly influences its density. In carbon allotropes, the arrangement of carbon atoms and the type of bonding (covalent in diamond, van der Waals in graphite) determine how closely packed the atoms are. Buckminsterfullerene's spherical structure suggests a lower density than diamond but potentially higher than graphite, as its unique geometry allows for a different packing efficiency.
Recommended video:
Guided course

Density Concepts
Related Practice
Textbook Question
Textbook Question
Germanium has the same structure as silicon, but the unit cell size is different because Ge and Si atoms are not the same size. If you were to repeat the experiment described in Additional Exercise 12.117, but replace the Si crystal with a Ge crystal, would you expect the X rays to be diffracted at a larger or smaller angle 𝜃?
Textbook Question
(b) X-ray diffraction studies of buckminsterfullerene show that it has a face-centered cubic lattice of C60 molecules. The length of an edge of the unit cell is 14.2 Å. Calculate the density of buckminsterfullerene.
