In their study of X-ray diffraction, William and Lawrence Bragg determined that the relationship among the wavelength of the radiation 1l2, the angle at which the radiation is diffracted 1u2, and the distance between planes of atoms in the crystal that cause the diffraction (d) is given by nl = 2d sin u. X rays from a copper X-ray tube that have a wavelength of 1.54 Å are diffracted at an angle of 14.22 degrees by crystalline silicon. Using the Bragg equation, calculate the distance between the planes of atoms responsible for diffraction in this crystal, assuming n = 1 (first-order diffraction).
Ch.12 - Solids and Modern Materials

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 12, Problem 118
Germanium has the same structure as silicon, but the unit cell size is different because Ge and Si atoms are not the same size. If you were to repeat the experiment described in Additional Exercise 12.117, but replace the Si crystal with a Ge crystal, would you expect the X rays to be diffracted at a larger or smaller angle 𝜃?
 Verified step by step guidance
Verified step by step guidance1
insert step 1> Understand that the problem involves X-ray diffraction, which is described by Bragg's Law: n\lambda = 2d\sin\theta, where n is the order of reflection, \lambda is the wavelength of the X-rays, d is the distance between atomic planes, and \theta is the angle of diffraction.
insert step 2> Recognize that the problem is asking about the change in the angle \theta when replacing a silicon (Si) crystal with a germanium (Ge) crystal.
insert step 3> Note that germanium atoms are larger than silicon atoms, which means the distance between atomic planes (d) in a Ge crystal is greater than in a Si crystal.
insert step 4> According to Bragg's Law, if the distance d increases and the wavelength \lambda remains constant, the angle \theta must adjust to satisfy the equation.
insert step 5> Conclude that with a larger d, the angle \theta will be smaller to maintain the equality in Bragg's Law, assuming the same order of reflection and wavelength.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Bragg's Law
Bragg's Law relates the angle of diffraction of X-rays to the spacing between the planes of atoms in a crystal. It is expressed as nλ = 2d sin(θ), where n is an integer, λ is the wavelength of the X-rays, d is the distance between atomic planes, and θ is the angle of diffraction. Understanding this relationship is crucial for predicting how changes in atomic spacing affect diffraction patterns.
Recommended video:
Guided course
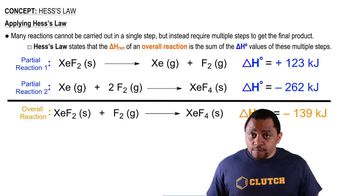
Hess's Law
Atomic Radius and Crystal Structure
The atomic radius is a measure of the size of an atom, which influences the arrangement of atoms in a crystal lattice. In the case of germanium (Ge) and silicon (Si), the difference in atomic radii affects the unit cell dimensions. A larger atomic radius typically results in a larger unit cell, which can lead to changes in the angles at which X-rays are diffracted.
Recommended video:
Guided course
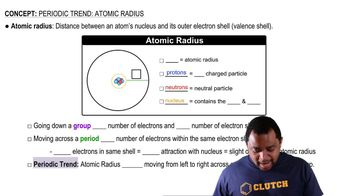
Atomic Radius
X-ray Diffraction
X-ray diffraction is a technique used to study the structure of crystalline materials by observing the angles and intensities of X-rays scattered by the crystal. The diffraction pattern provides information about the arrangement of atoms within the crystal. The angle of diffraction is influenced by the crystal structure and the wavelength of the X-rays, making it essential to consider these factors when analyzing different materials.
Recommended video:
Guided course
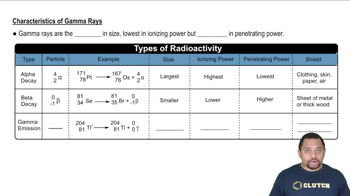
Characteristics of Gamma Rays
Related Practice
Textbook Question
Textbook Question
(a) The density of diamond is 3.5 g/cm3, and that of graphite is 2.3 g/cm3. Based on the structure of buckminsterfullerene, what would you expect its density to be relative to these other forms of carbon?
Textbook Question
(b) X-ray diffraction studies of buckminsterfullerene show that it has a face-centered cubic lattice of C60 molecules. The length of an edge of the unit cell is 14.2 Å. Calculate the density of buckminsterfullerene.
