Osmosis is a vital biological process defined as the passive diffusion of water across a semi-permeable membrane, such as a cell membrane. This process does not require energy, distinguishing it from active transport mechanisms. In osmosis, the solvent, primarily water in biological contexts, moves in response to solute concentrations on either side of the membrane.
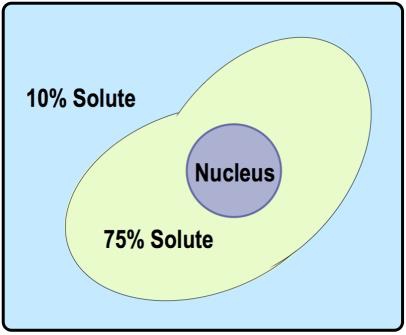
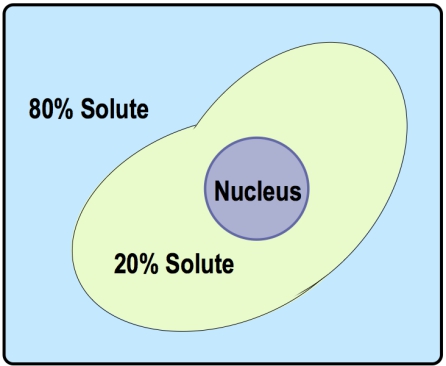
The concept of tonicity is crucial in understanding osmosis, as it describes the relative concentration of solutes in a solution. Tonicity can be categorized into three key terms: hypotonic, isotonic, and hypertonic. A hypotonic solution has a lower solute concentration compared to another solution, which can be remembered by the prefix "hypo," meaning low. Conversely, a hypertonic solution has a higher solute concentration, indicated by the prefix "hyper," which signifies excess. An isotonic solution has equal solute concentrations on both sides of the membrane, with "iso" meaning equal.
These terms are comparative; they only make sense when discussing two regions, typically the inside and outside of a cell. For example, if the outside solution has a lower concentration of solutes than the inside, it is labeled hypotonic, while the inside is hypertonic. If the concentrations are equal, both are isotonic. Understanding these relationships is essential for predicting the direction of water flow during osmosis, which will be explored further in subsequent discussions.