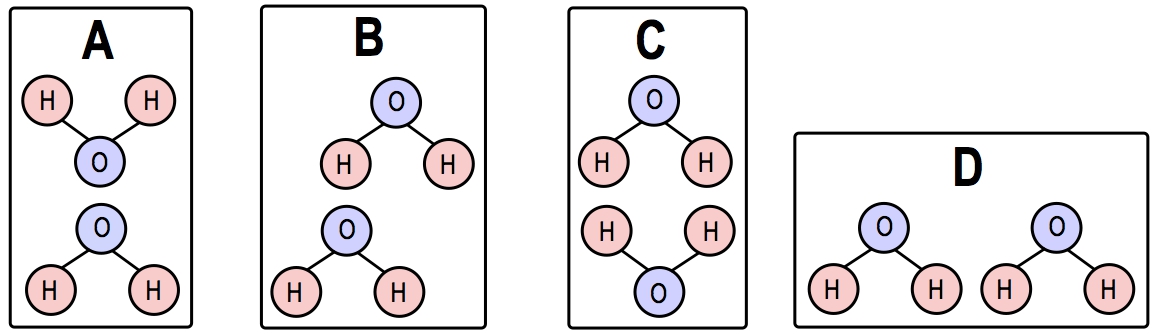
Hydrogen bonding, often abbreviated as H bond, is a crucial interaction in chemistry and biology. It occurs between a hydrogen atom and a highly electronegative atom, typically fluorine, oxygen, or nitrogen. These electronegative atoms are essential for forming hydrogen bonds, and a helpful mnemonic to remember them is "FON," which sounds like "fun." Each hydrogen bond involves a hydrogen atom, making it a fundamental component of the interaction.
Individually, hydrogen bonds are relatively weak; however, when many hydrogen bonds form collectively, they can create significant strength. This property is particularly important in biological contexts, such as the unique characteristics of water and the structure of macromolecules. For instance, water molecules (H2O) can form hydrogen bonds with each other, where the bond is represented by the interaction between a hydrogen atom and an oxygen atom. These hydrogen bonds contribute to water's essential properties, which are vital for life.
Additionally, hydrogen bonds play a significant role in the structure of nucleotides, the building blocks of DNA. In this context, hydrogen bonds can form between hydrogen atoms and electronegative atoms like oxygen and nitrogen within nucleotides. Understanding these interactions is crucial for grasping the structure and function of DNA, which will be explored further in later discussions.
In summary, hydrogen bonds are weak interactions that become strong when numerous bonds form together, influencing various biological processes and the properties of substances like water. This foundational knowledge sets the stage for deeper exploration of hydrogen bonding in biological macromolecules and their significance in life sciences.