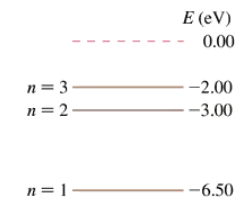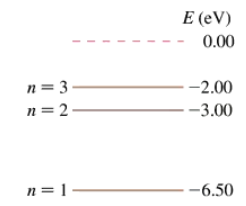Back
BackProblem 52
An electron confined in a one-dimensional box emits a 200 nm photon in a quantum jump from n = 2 to n = 1. What is the length of the box?
Problem 54a
The first three energy levels of the fictitious element X are shown in FIGURE P38.54. What is the ionization energy of element X?
Problem 54b
The first three energy levels of the fictitious element X are shown in FIGURE P38.54. What wavelengths are observed in the absorption spectrum of element X? Express your answers in nm.
Problem 55b
The absorption spectrum of an atom consists of the wavelengths 200 nm, 300 nm, and 500 nm. b. What wavelengths are seen in the atom’s emission spectrum?
Problem 56
The first three energy levels of the fictitious element X were shown in Figure P38.54. An electron with a speed of 1.4×106 m/s collides with an atom of element X. Shortly afterward, the atom emits a photon with a wavelength of 1240 nm. What was the electron’s speed after the collision? Assume that, because the atom is much more massive than the electron, the recoil of the atom is negligible. Hint: The energy of the photon is not the energy transferred to the atom in the collision.
Problem 61a
What wavelength photon does a hydrogen atom emit in a 200→199 transition?
Problem 61b
What is the difference in the wavelengths emitted in a 199→2 transition and a 200→2 transition?
Problem 62b
Consider a hydrogen atom in stationary state n. On average, an atom stays in the n = 2 state for 1.6 ns before undergoing a transition to the n = 1 state. On average, how many revolutions does the electron make before the transition?
Problem 63a
Draw an energy-level diagram, similar to Figure 38.21, for the He+ ion. On your diagram: Show the first five energy levels. Label each with the values of n and En.
Problem 63b
Draw an energy-level diagram, similar to Figure 38.21, for the He+ ion. On your diagram: Show the ionization limit.
Problem 63c
Draw an energy-level diagram, similar to Figure 38.21, for the He+ ion. On your diagram: Show all possible emission transitions from the n = 4 energy level.
Problem 64
Very large, hot stars—much hotter than our sun—can be identified by the way in which He+ ions in their atmosphere absorb light. What are the three longest wavelengths, in nm, in the Balmer series of He+?
Problem 67
INT Two hydrogen atoms collide head-on. The collision brings both atoms to a halt. Immediately after the collision, both atoms emit a 121.6 nm photon. What was the speed of each atom just before the collision?
Problem 68a
INT A beam of electrons is incident upon a gas of hydrogen atoms. What minimum speed must the electrons have to cause the emission of 656 nm light from the 3→2 transition of hydrogen?
Problem 68b
INT A beam of electrons is incident upon a gas of hydrogen atoms. Through what potential difference must the electrons be accelerated to have this speed?
Problem 69
The electrons in a cathode-ray tube are accelerated through a 250 V potential difference and then shot through a 33-nm-diameter circular aperture. What is the diameter of the bright spot on an electron detector 1.5 m behind the aperture?
Problem 71d
In the atom interferometer experiment of Figure 38.13, laser-cooling techniques were used to cool a dilute vapor of sodium atoms to a temperature of 0.0010 K=1.0 mK. The ultracold atoms passed through a series of collimating apertures to form the atomic beam you see entering the figure from the left. The standing light waves were created from a laser beam with a wavelength of 590 nm. Because interference is observed between the two paths, each individual atom is apparently present at both point B and point C. Describe, in your own words, what this experiment tells you about the nature of matter.
Problem 72b
Consider an electron undergoing cyclotron motion in a magnetic field. According to Bohr, the electron’s angular momentum must be quantized in units of ℏ. Compute the first four allowed radii in a 1.0 T magnetic field.
Problem 72c
Consider an electron undergoing cyclotron motion in a magnetic field. According to Bohr, the electron’s angular momentum must be quantized in units of ℏ. Find an expression for the allowed energy levels En in terms of ℏ and the cyclotron frequency fcyc.