Back
BackProblem 3
Electrons in a photoelectric-effect experiment emerge from an aluminum surface with a maximum kinetic energy of 1.30 eV. What is the wavelength of the light?
Problem 4
Photoelectrons are observed when a metal is illuminated by light with a wavelength less than 388 nm. What is the metal's work function?
Problem 6a
A photoelectric-effect experiment finds a stopping potential of 1.56 V when light of 200 nm is used to illuminate the cathode. From what metal is the cathode made?
Problem 8
What is the wavelength, in nm, of a photon with energy (a) 0.30 eV, (b) 3.0 eV, and (c) 30 eV? For each, is this wavelength visible, ultraviolet, or infrared light?
Problem 13
A 100 W incandescent lightbulb emits about 5 W of visible light. (The other 95 W are emitted as infrared radiation or lost as heat to the surroundings.) The average wavelength of the visible light is about 600 nm, so make the simplifying assumption that all the light has this wavelength. How many visible-light photons does the bulb emit per second?
Problem 14
What is the energy, in keV, of 75 keV x-ray photons that are backscattered (i.e., scattered directly back toward the source) by the electrons in a target?
Problem 15
55 keV x-ray photons are incident on a target. At what scattering angle do the scattered photons have an energy of 50 keV?
Problem 16
At what speed is an electron’s de Broglie wavelength (a) 1.0 nm, (b) 1.0 μm, and (c) 1.0 mm?
Problem 17
INT Through what potential difference must an electron be accelerated from rest to have a de Broglie wavelength of 500 nm?
Problem 19a
What is the de Broglie wavelength of a 200 g baseball with a speed of 30 m/s?
Problem 20
The diameter of the nucleus is about 10 fm. What is the kinetic energy, in MeV, of a proton with a de Broglie wavelength of 10 fm?
Problem 21
What is the quantum number of an electron confined in a 3.0-nm-long one-dimensional box if the electron’s de Broglie wavelength is 1.0 nm?
Problem 23
The diameter of the nucleus is about 10 fm. A simple model of the nucleus is that protons and neutrons are confined within a one-dimensional box of length 10 fm. What are the first three energy levels, in MeV, for a proton in such a box?
Problem 26
The allowed energies of a simple atom are 0.00 eV, 4.00 eV, and 6.00 eV. An electron traveling with a speed of 1.30×106 m/s collides with the atom. Can the electron excite the atom to the n = 2 stationary state? The n = 3 stationary state? Explain.
Problem 27a
The allowed energies of a simple atom are 0.00 eV, 4.00 eV, and 6.00 eV. Draw the atom’s energy-level diagram. Label each level with the energy and the quantum number.
Problem 27b
The allowed energies of a simple atom are 0.00 eV, 4.00 eV, and 6.00 eV. What wavelengths appear in the atom’s emission spectrum?
Problem 27c
The allowed energies of a simple atom are 0.00 eV, 4.00 eV, and 6.00 eV. What wavelengths appear in the atom’s absorption spectrum?
Problem 30
What is the radius of a hydrogen atom whose electron moves at 7.3×105 m/s?
Problem 31a
What quantum number of the hydrogen atom comes closest to giving a 100-nm-diameter electron orbit?
Problem 34
Find the radius of the electron’s orbit, the electron’s speed, and the energy of the atom for the first three stationary states of He+.
Problem 36b
A ruby laser emits an intense pulse of light that lasts a mere 10 ns. The light has a wavelength of 690 nm, and each pulse has an energy of 500 mJ. What is the rate of photon emission, in photons per second, during the 10 ns that the laser is 'on'?
Problem 38
BIO The wavelengths of light emitted by a firefly span the visible spectrum but have maximum intensity near 550 nm. A typical flash lasts for 100 ms and has a power output of 1.2 mW. How many photons does a firefly emit in one flash if we assume that all light is emitted at the peak intensity wavelength of 550 nm?
Problem 41a
Potassium and gold cathodes are used in a photoelectric-effect experiment. For each cathode, find: The threshold frequency.
Problem 41d
Potassium and gold cathodes are used in a photoelectric-effect experiment. For each cathode, find: The stopping potential if the wavelength is 220 nm.
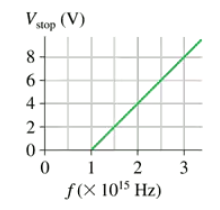
Problem 42a
The graph in FIGURE P38.42 was measured in a photoelectric-effect experiment. What is the work function (in eV) of the cathode?
Problem 44
A 75 kW radio transmitter emits 550 kHz radio waves uniformly in all directions. At what rate do photons strike a 1.5-m-tall, 3.0-mm-diameter antenna that is 15 km away?
Problem 45
The cosmic microwave background radiation is light left over from the Big Bang that has been Doppler-shifted to microwave frequencies by the expansion of the universe. It now fills the universe with 450 photons/cm3 at an average frequency of 160 GHz. How much energy from the cosmic microwave background, in MeV, fills a small apartment that has 95 m2 of floor space and 2.5-m-high ceilings?
Problem 47b
INT The electron interference pattern of Figure 38.12 was made by shooting electrons with 50 keV of kinetic energy through two slits spaced 1.0 μm apart. The fringes were recorded on a detector 1.0 m behind the slits. Figure 38.12 is greatly magnified. What was the actual spacing on the detector between adjacent bright fringes?
Problem 49
An electron confined in a one-dimensional box is observed, at different times, to have energies of 12 eV, 27 eV, and 48 eV. What is the length of the box?
Problem 50
A muon—a subatomic particle with charge −e and a mass 207 times that of an electron—is confined in a 15-pm-long, one-dimensional box. ( 1 pm=1 picometer=10−12 m.) What is the wavelength, in nm, of the photon emitted in a quantum jump from n = 2 to n = 1?