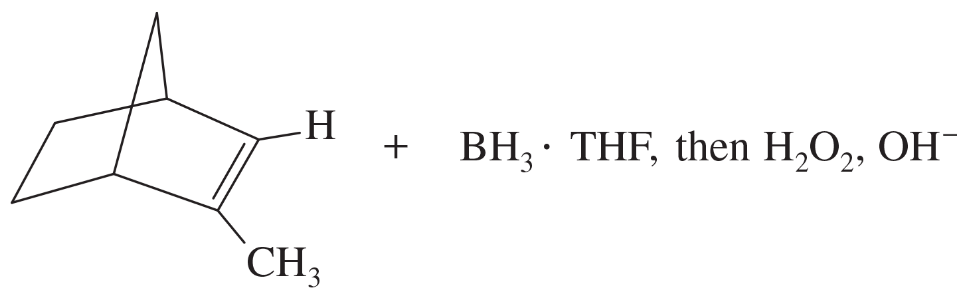Back
BackProblem 11b
Show how you would accomplish the following synthetic conversions.
(b) but-1-ene → butan-2-ol
Problem 11c
Show how you would accomplish the following synthetic conversions.
c. 2-bromo-2,4-dimethylpentane → 2,4-dimethylpentan-3-ol
Problem 12
In the hydroboration of 1-methylcyclopentene shown in Solved Problem 8-3, the reagents are achiral, and the products are chiral. The product is a racemic mixture of trans-2-methylcyclopentanol, but only one enantiomer is shown. Show how the other enantiomer is formed.
Problem 13a
Predict the major products of the following reactions. Include stereochemistry where applicable.
(a) 1−methylcyclohexene + BH3⋅THF then H2O2, OH–
Problem 13b
Predict the major products of the following reactions. Include stereochemistry where applicable.
(b) trans-4,4-dimethylpent-2-ene + BH3⋅THF then H2O2, OH–
Problem 13c
Predict the major products of the following reactions. Include stereochemistry where applicable.
(c)
Problem 14a
When (Z)-3-methylhex-3-ene undergoes hydroboration–oxidation, two isomeric products are formed. Give their structures, and label each asymmetric carbon atom as (R) or (S). What is the relationship between these isomers?
Problem 14b
When (E)-3-methylhex-3-ene undergoes hydroboration–oxidation, two isomeric products are formed. Give their structures, and label each asymmetric carbon atom as (R) or (S). What is the relationship between these isomers? What is the relationship between the products formed from (Z)-3-methylhex-3-ene and those formed from (E)-3-methylhex-3-ene?
Problem 15a
Show how you would accomplish the following transformations.
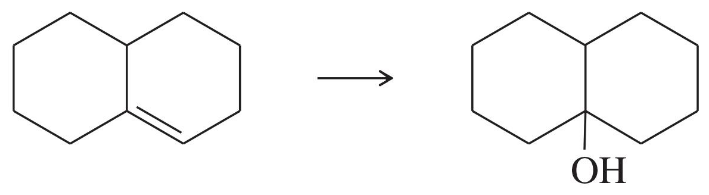
(a)
Problem 15b
Show how you would accomplish the following transformations.
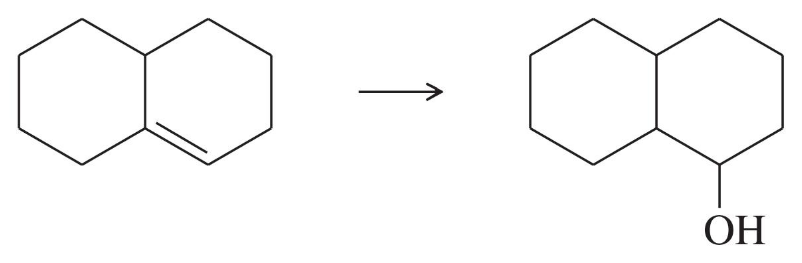
(b)
Problem 15c
Show how you would accomplish the following transformations.
(c) 1-methylcycloheptanol → 2-methylcycloheptanol
Problem 16a
When HBr adds across the double bond of 1,2-dimethylcyclopentene, the product is a mixture of the cis and trans isomers. Show why this addition is not stereospecific.
Problem 16b
When 1,2-dimethylcyclopentene undergoes hydroboration–oxidation, one diastereomer of the product predominates. Show why this addition is stereospecific, and predict the stereochemistry of the major product.
Problem 17
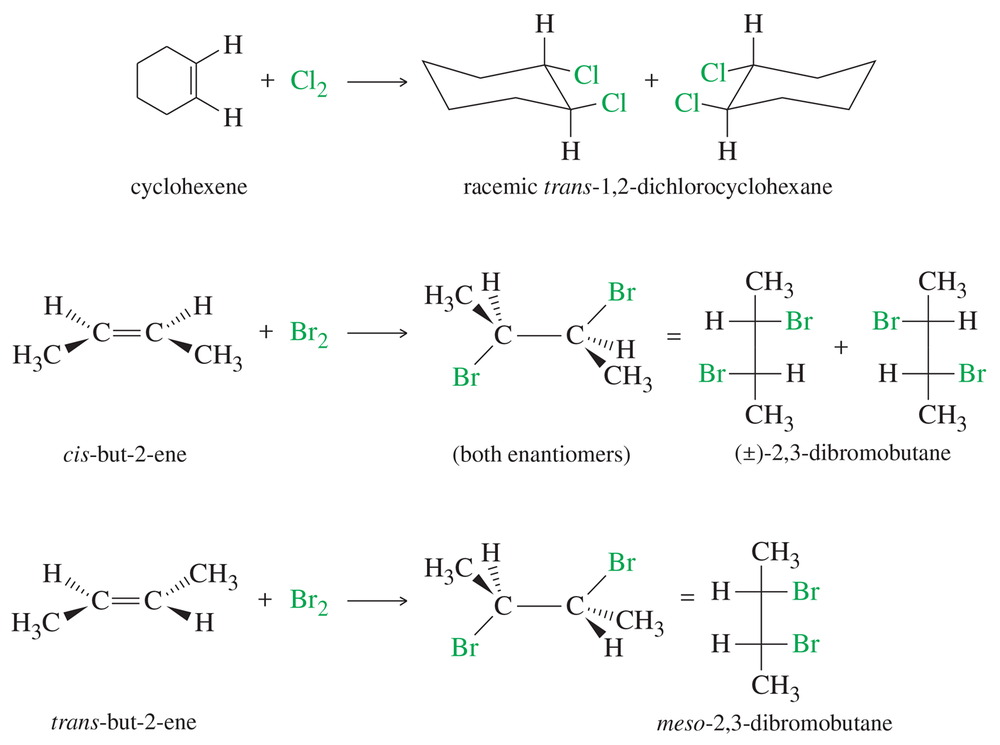
Give mechanisms to account for the stereochemistry of the products observed from the addition of bromine to cis- and trans-but-2-ene (Figure 8-5). Why are two products formed from the cis isomer but only one from the trans? (Making models will be helpful.)
Problem 18c,d
Propose mechanisms and predict the major products of the following reactions. Include stereochemistry where appropriate.
(c) (E)-dec-3-ene + Br2 in CCl4
(d) (Z)-dec-3-ene + Br2 in CCl4
Problem-Solving Hint: Models may be helpful whenever stereochemistry is involved. Write complete structures, including all bonds and charges, when writing mechanisms.
Problem 19
Propose a mechanism for the addition of bromine water to cyclopentene, being careful to show why the trans product results and how both enantiomers are formed.
Problem 20a
The solutions to Solved Problem 8-5 showed only how one enantiomer of the product is formed. For each product, show how an equally probable reaction forms the other enantiomer.
Problem 20b
The solutions to Solved Problem 8-6 showed only how one enantiomer of the product is formed. For each product, show how an equally probable reaction forms the other enantiomer.
Problem 21a,b
Predict the major product(s) for each reaction. Include stereochemistry where appropriate.
a. 1-methylcyclohexene + Cl2/H2O
b. 2-methylbut-2-ene + Br2/H2O
Problem 21c,d
Predict the major product(s) for each reaction. Include stereochemistry where appropriate.
c. cis-but-2-ene + Cl2/H2O
d. trans-but-2-ene + Cl2/H2O
Problem 21e
Predict the major product(s) for each reaction. Include stereochemistry where appropriate.
e. 1-methylcyclopentene + Br2 in saturated aqueous NaCl
Problem 22a
Show how you would accomplish the following synthetic conversions.
a. 3-methylpent-2-ene → 2-chloro-3-methylpentan-3-ol
Problem-Solving Hint: The opening of a halonium ion is driven by its electrophilic nature. The weak nucleophile attacks the carbon bearing more positive charge.
Problem 22b
Show how you would accomplish the following synthetic conversions.
b. chlorocyclohexane → trans-2-chlorocyclohexanol
Problem-Solving Hint: The opening of a halonium ion is driven by its electrophilic nature. The weak nucleophile attacks the carbon bearing more positive charge.
Problem 22c
Show how you would accomplish the following synthetic conversions.
c. 1-methylcyclopentanol → 2-chloro-1-methylcyclopentanol
Problem-Solving Hint: The opening of a halonium ion is driven by its electrophilic nature. The weak nucleophile attacks the carbon bearing more positive charge.
Problem 23a,b
Give the expected major product for each reaction, including stereochemistry where applicable.
(a) but-1-ene + H2/Pt
(b) cis-but-2-ene + H2/Ni
Problem 23c,d
Give the expected major product for each reaction, including stereochemistry where applicable.
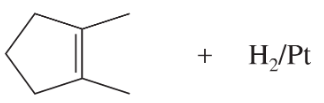
(c)
(d)
Problem 24a
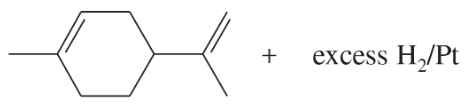
One of the principal components of lemongrass oil is limonene, C10H16. When limonene is treated with excess hydrogen and a platinum catalyst, the product is an alkane of formula C10H20. What can you conclude about the structure of limonene?
Problem 25
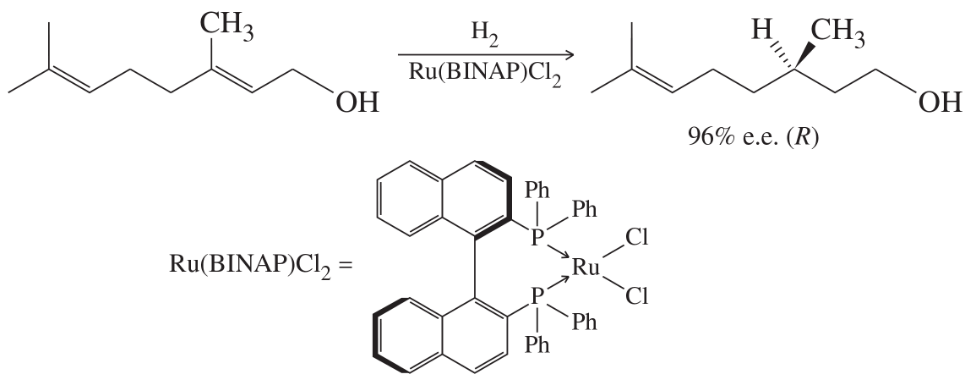
The chiral BINAP ligand shown in Figure 8-8 contains no asymmetric carbon atoms. Explain how this ligand is chiral.
Problem 26
Predict the carbenoid addition products of the following reactions.
a. trans-hex-3-ene + CH2I2, Zn(Cu)
b. cis-hex-3-ene + CH2I2, Zn(Cu)
Problem 27a
Predict the carbenoid addition products of the following reactions.
(a) cyclohexene + CHCl3, 50% NaOH/H2O