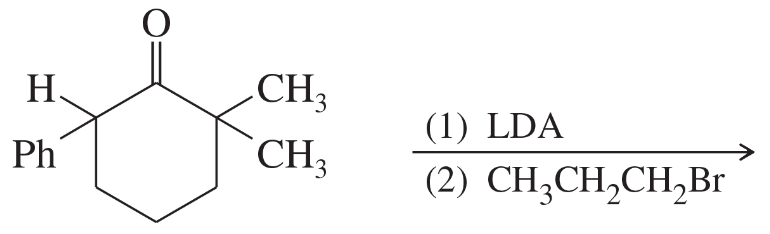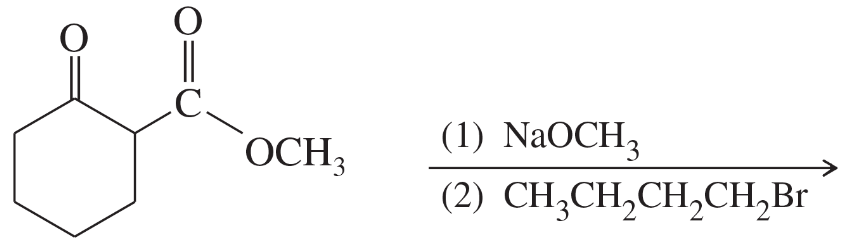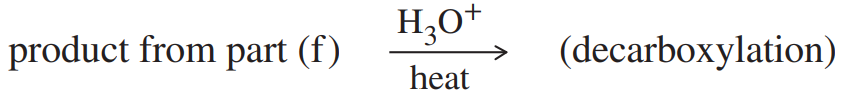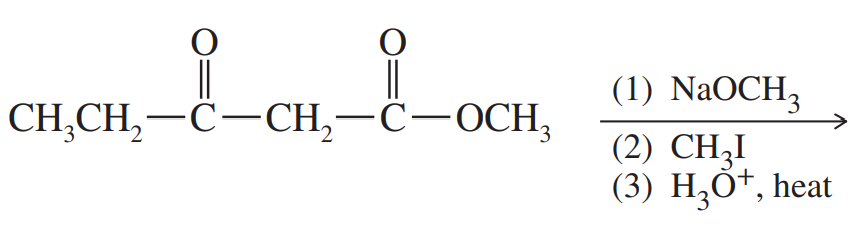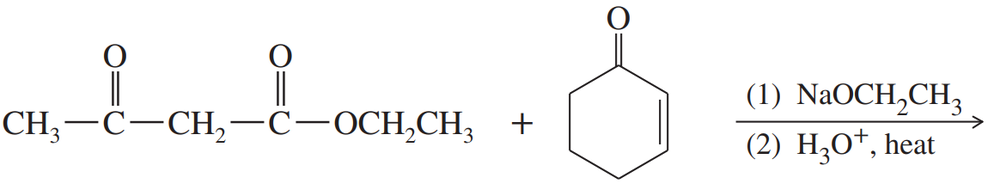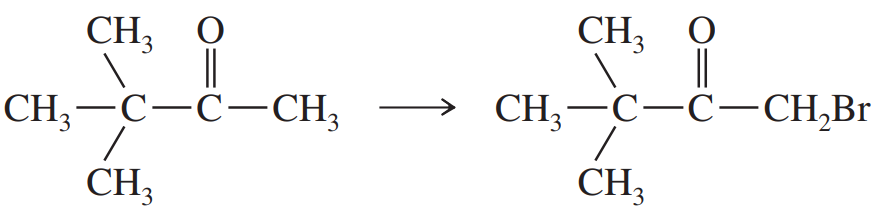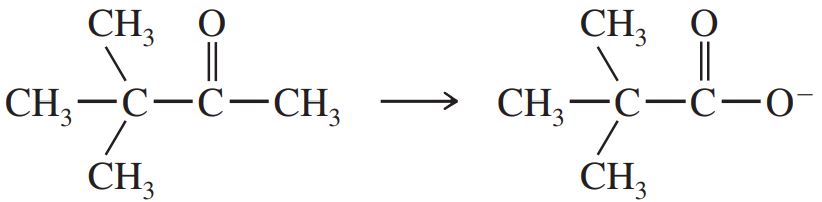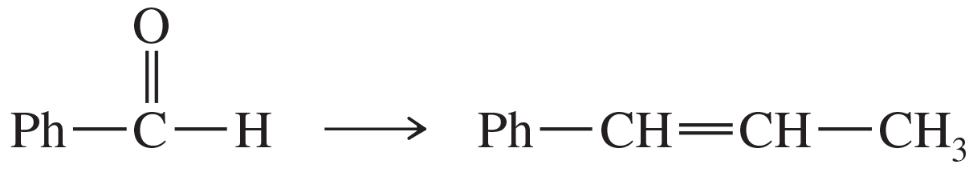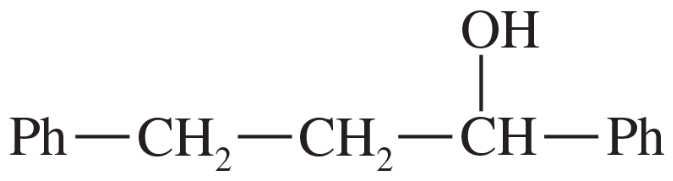Back
BackProblem 66a
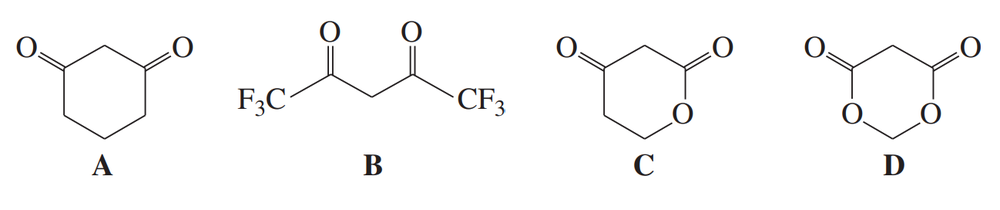
Rank these compounds in order of increasing acid strength.
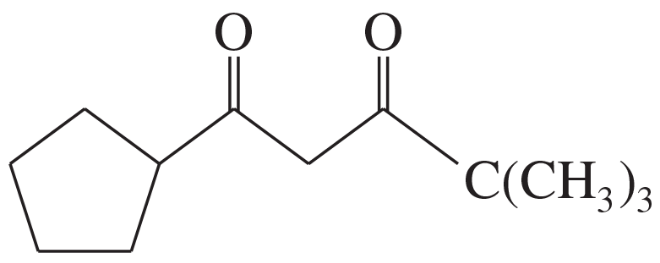
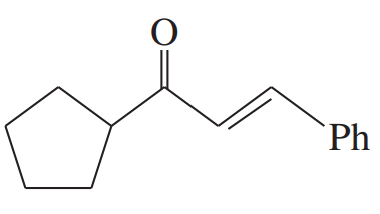
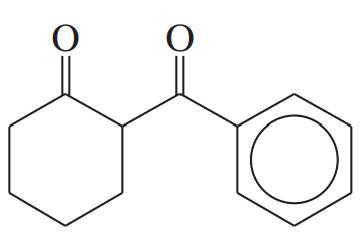
Problem 66b
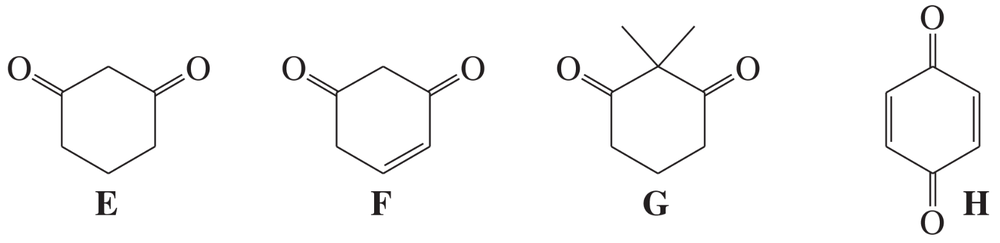
Rank these compounds in order of increasing enol content. In each case, draw the most stable enol.
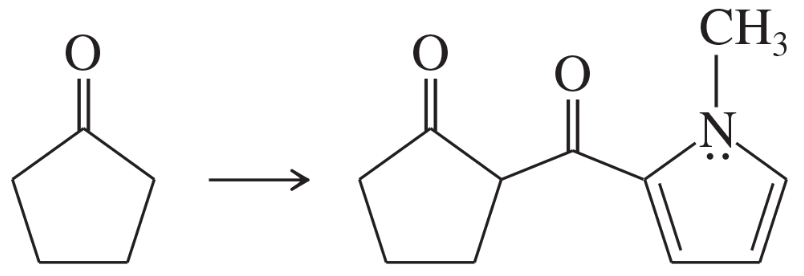
Problem 67a
Show how you would use the Robinson annulation to synthesize the following compounds.
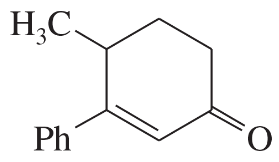
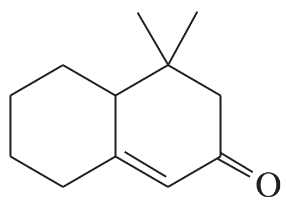
(a)
Problem 67b
Show how you would use the Robinson annulation to synthesize the following compounds.
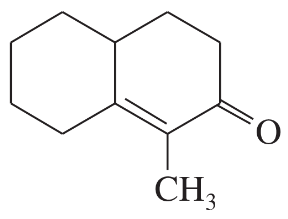
(b)
Problem 67c
Show how you would use the Robinson annulation to synthesize the following compounds.
(c)
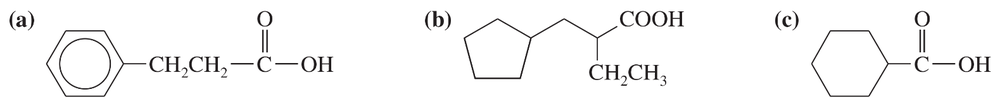
Problem 68a
Show how you would use an aldol, Claisen, or another type of condensation to make each compound.
(a)
Problem 68b
Show how you would use an aldol, Claisen, or another type of condensation to make each compound.
(b)
Problem 68c
Show how you would use an aldol, Claisen, or another type of condensation to make each compound.
(c)
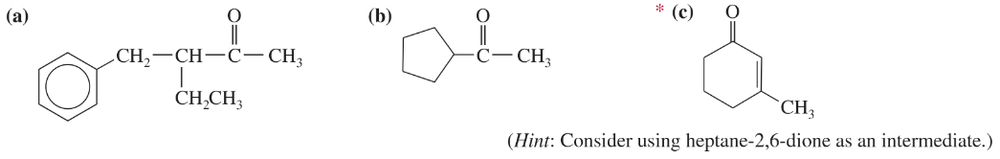
Problem 68d
Show how you would use an aldol, Claisen, or another type of condensation to make each compound.
(d)
Problem 68e
Show how you would use an aldol, Claisen, or another type of condensation to make each compound.
(e)
Problem 68f
Show how you would use an aldol, Claisen, or another type of condensation to make each compound.
(f)
Problem 69a
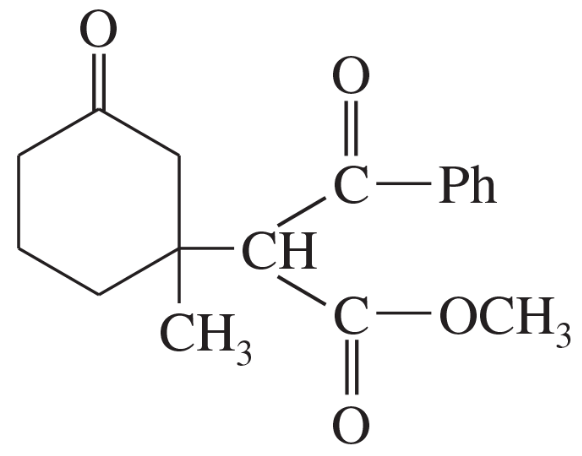
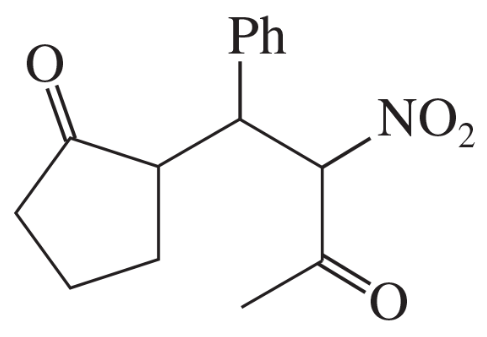
Predict the products of the following reactions.
(a) cyclopentanone + Br2 in acetic acid
Problem 69b
Predict the products of the following reactions.
(b) 1-phenylethanol + excess I2 in base
Problem 69c
Predict the products of the following reactions.
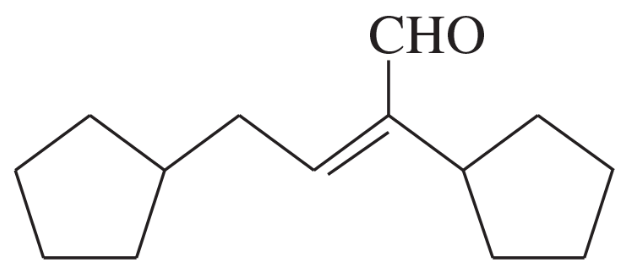
(c)
Problem 69d
Predict the products of the following reactions.
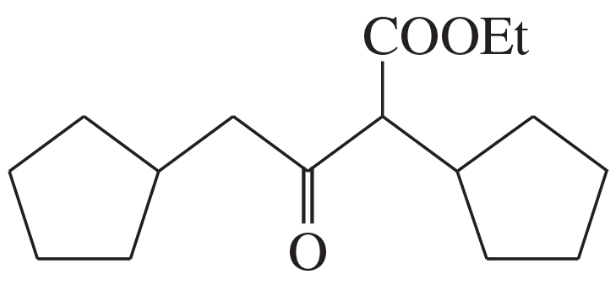
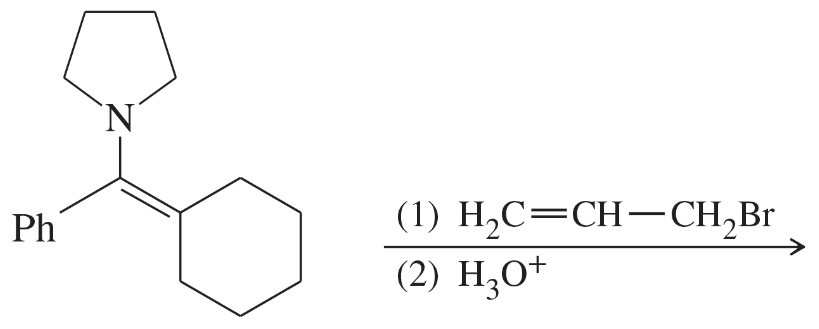
(d)
Problem 69e
Predict the products of the following reactions.
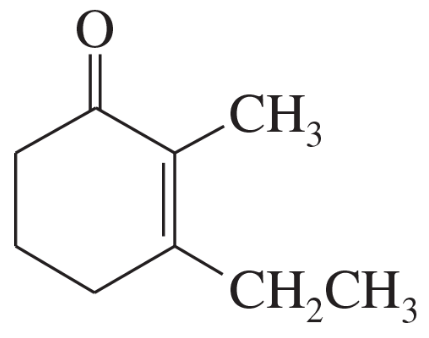
(e)
Problem 69f
Predict the products of the following reactions.
(f)
Problem 69g
Predict the products of the following reactions.
(g)
Problem 69h
Predict the products of the following reactions.
(h)
Problem 69i
Predict the products of the following reactions.
(i)
Problem 70
Predict the products of these reaction sequences.
Problem 71a
Show how you would accomplish the following conversions in good yields. You may use any necessary reagents.
(a)
Problem 71b
Show how you would accomplish the following conversions in good yields. You may use any necessary reagents.
(b)
Problem 71c
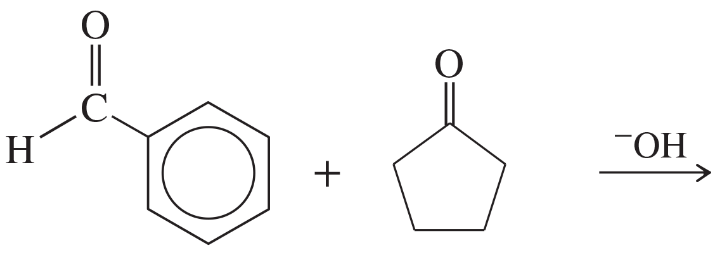
Show how you would accomplish the following conversions in good yields. You may use any necessary reagents.
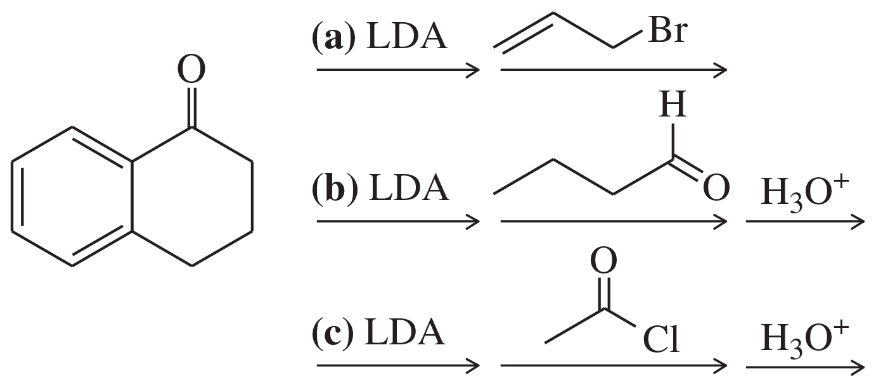
(c)
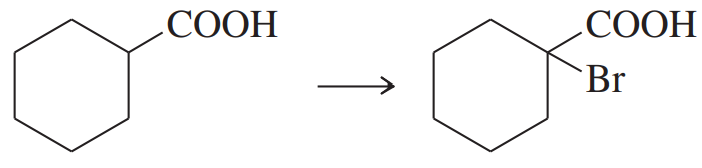
Problem 71d,e,f
Show how you would accomplish the following conversions in good yields. You may use any necessary reagents.
(d)
(e)
(f)
Problem 72
Show how you would use the malonic ester synthesis to make the following compounds.
Problem 73
Show how you would use the acetoacetic ester synthesis to make the following compounds.
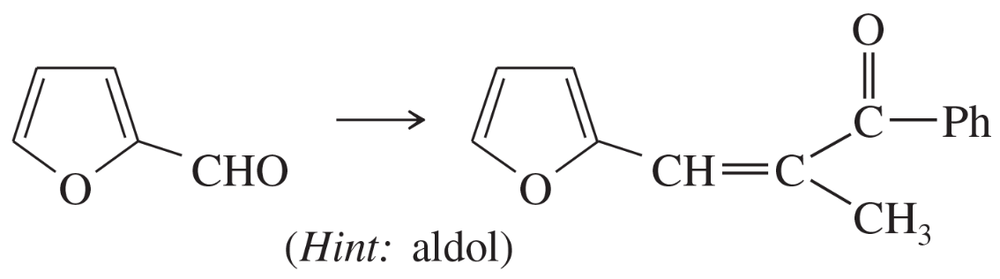
Problem 74a
The following compounds can be synthesized by aldol condensations, followed by further reactions. (In each case, work backward from the target molecule to an aldol product, and show what compounds are needed for the condensation.)
(a)
Problem 74b
The following compounds can be synthesized by aldol condensations, followed by further reactions. (In each case, work backward from the target molecule to an aldol product, and show what compounds are needed for the condensation.)
(b)
Problem 74c
The following compounds can be synthesized by aldol condensations, followed by further reactions. (In each case, work backward from the target molecule to an aldol product, and show what compounds are needed for the condensation.)
(c)