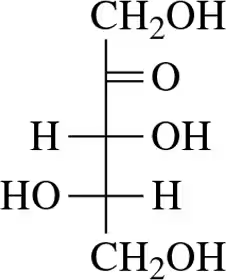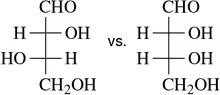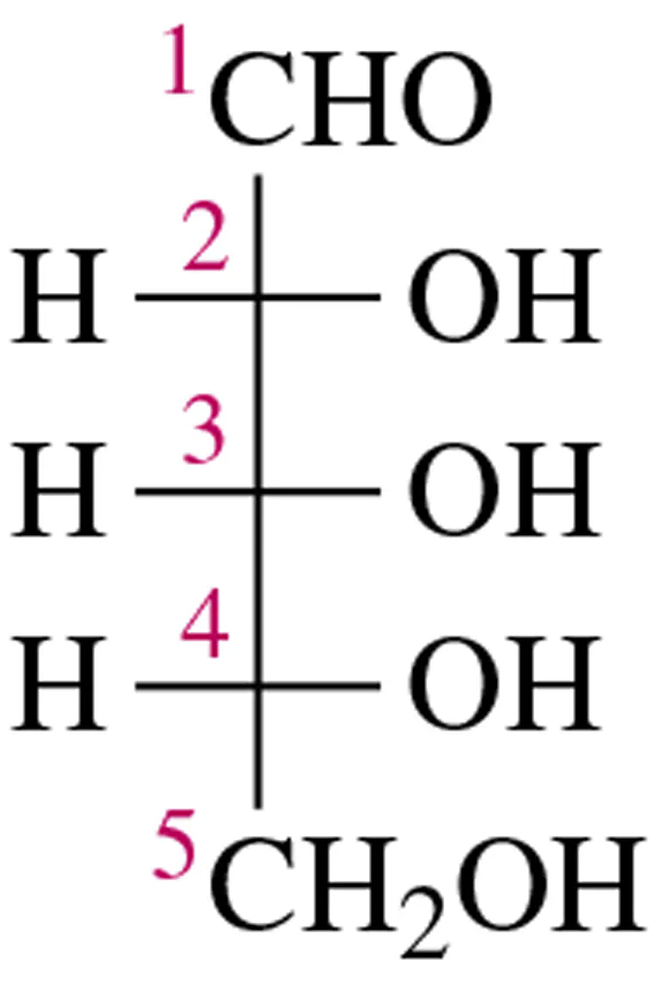Back
BackProblem 2a
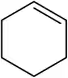
Identify the following alkenes as E or Z, if appropriate.
(a)
Problem 2b
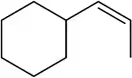
Identify the following alkenes as E or Z, if appropriate.
(b)
Problem 2c
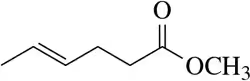
Identify the following alkenes as E or Z, if appropriate.
(c)
Problem 2d
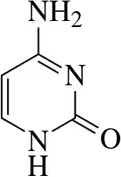
Identify the following alkenes as E or Z, if appropriate.
(d)
Problem 3a
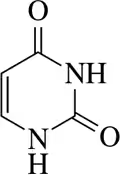
Identify the hydrogen bond donors and hydrogen bond acceptors in the following molecules.
(a)
Problem 3b
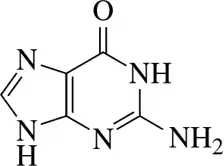
Identify the hydrogen bond donors and hydrogen bond acceptors in the following molecules.
(b)
Problem 3d
Identify the hydrogen bond donors and hydrogen bond acceptors in the following molecules.
(d)
Problem 4b
Predict the product of the following reactions.
(b)
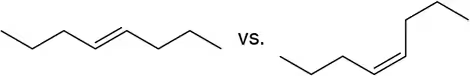
Problem 5a
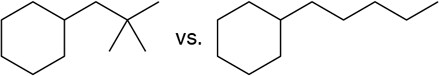
In each pair, which would you expect to have the higher melting point?
(a)
Problem 5b
In each pair, which would you expect to have the higher melting point?
(b)
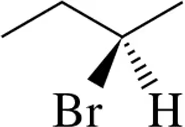
Problem 7a
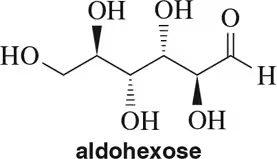
Draw Fischer projections of the following molecules.
(a)
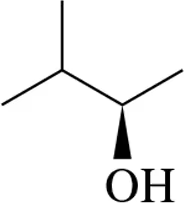
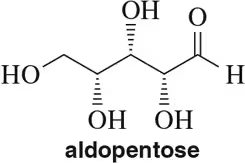
Problem 7b
Draw Fischer projections of the following molecules.
(b)
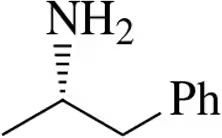
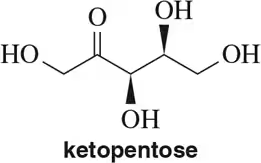
Problem 7c
Draw Fischer projections of the following molecules.
(c)
Problem 8
In this attempt to convert the line angle drawing of d-erythrose (shown) to the Fischer projection (shown), by viewing it from a certain direction (shown), a mistake was made. What was the mistake?
<IMAGE>
Problem 9a
Convert each line-angle drawing, using appropriate bond rotations, into a correct Fischer projection.
(a)
Problem 9b
Convert each line-angle drawing, using appropriate bond rotations, into a correct Fischer projection.
(b)
Problem 9c
Convert each line-angle drawing, using appropriate bond rotations, into a correct Fischer projection.
(c)
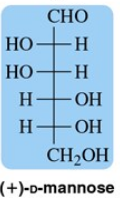
Problem 10a
Categorize the following monosaccharides as d or l.
(a)
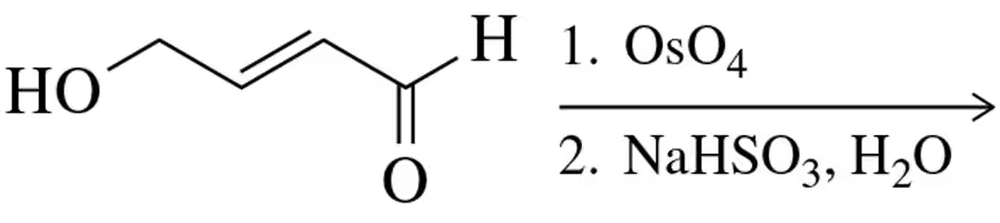
Problem 12
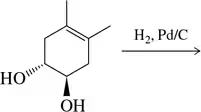
Draw the structure of and name the enantiomeric diols that result from the cis-dihydroxylation of the alkene shown.
Problem 14
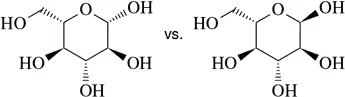
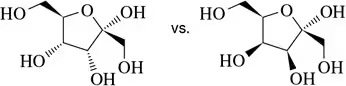
Which of the following molecule pairs are epimers?
(a)
(b)
(c)
Problem 15
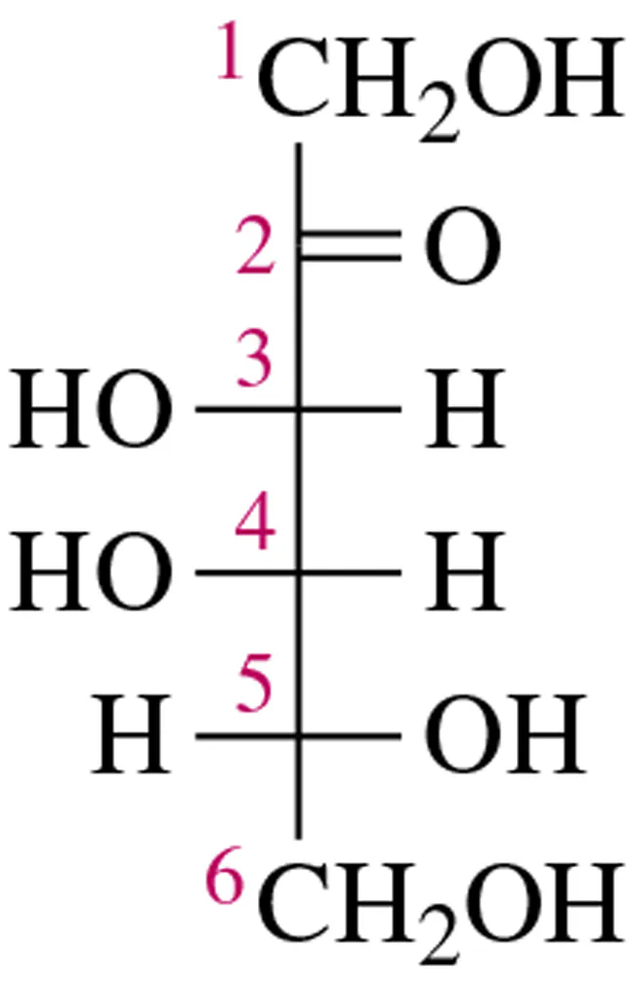
Using the Haworth projection, draw the furanose ring that would form between the C5 hydroxyl group and the ketone at C2 for the molecule shown.
Problem 16
Using the Haworth projection, draw the furanose ring that would form between the C4 hydroxyl group and the aldehyde at C1 for the molecule shown.
Problem 22
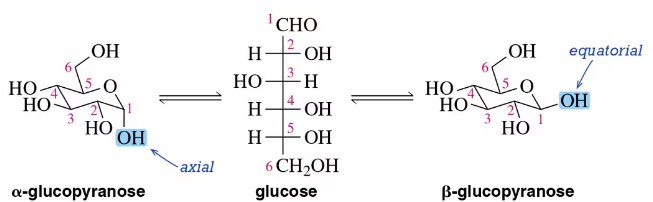
Suggest a mechanism by which α-d-glucopyranose is converted to β-d-glucopyranose in acid. [See Figure 27.18.]
Problem 23
Suggest a mechanism by which α-d-glucopyranose is converted to β-d-glucopyranose in base. [See Figure 27.18.]
Problem 24
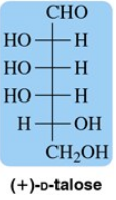
Draw the α- and β-anomers of d-talopyranose. [The structure of talose is in Figure 27.11.]
Problem 25
Draw the α- and β-anomers of d-mannofuranose. [The structure of mannose is in Figure 27.11.]
Problem 26b
Draw the structure that corresponds to the given name.
(b) Benzyl α-d-gulopyranoside
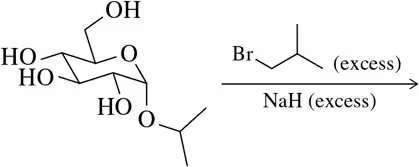
Problem 30a
Predict the product of the following etherification reactions.
(a)
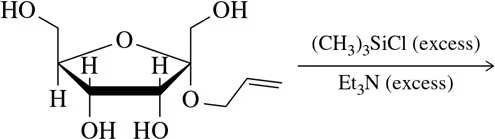
Problem 30b
Predict the product of the following etherification reactions.
(b)
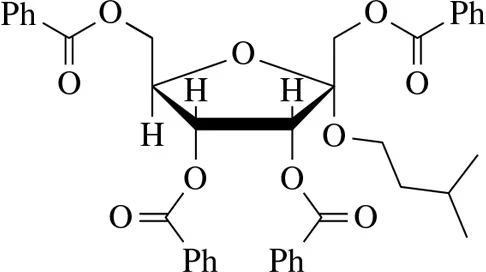
Problem 32a
Suggest a synthesis of the following acylated sugars.
(a)