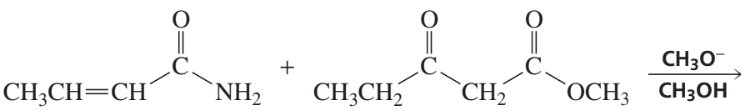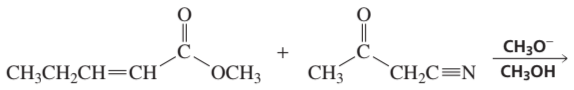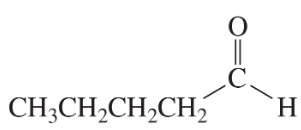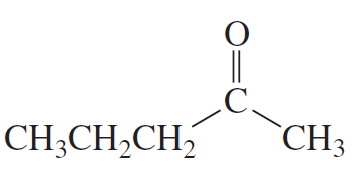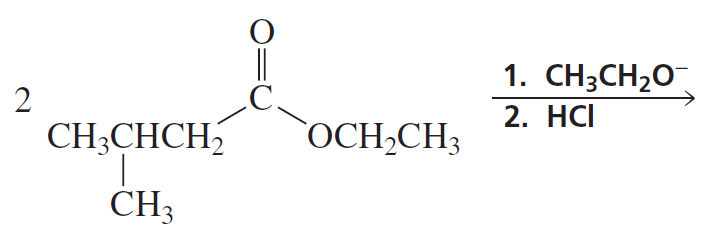Back
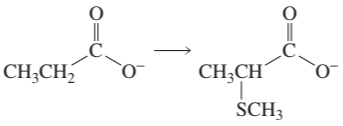
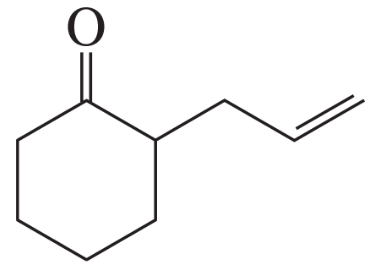
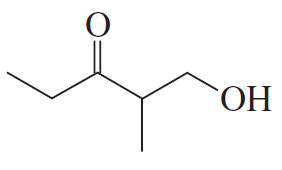
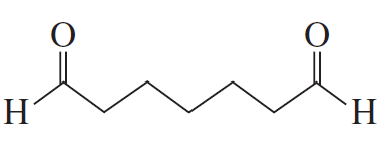
BackProblem 11a
Show how the following compounds can be prepared from the given starting material:
a.
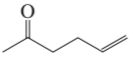
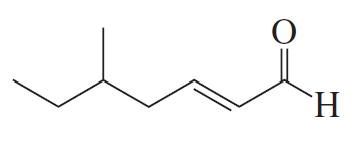
Problem 14a
How could each of the following compounds be prepared from a ketone and an alkyl halide?
a.
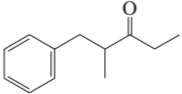
Problem 14b
How could each of the following compounds be prepared from a ketone and an alkyl halide?
b.
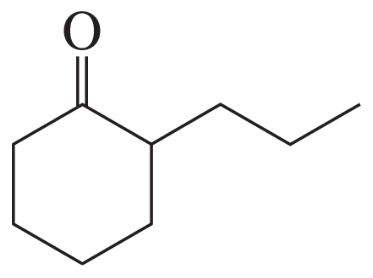
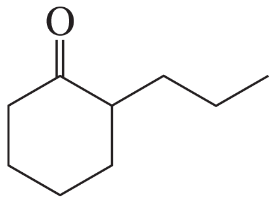
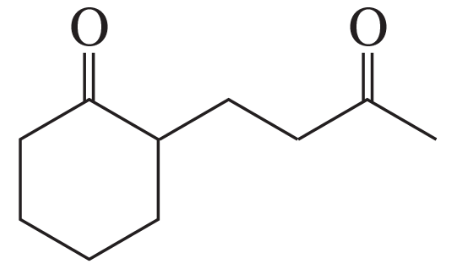
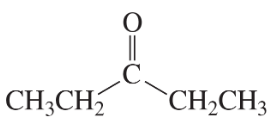
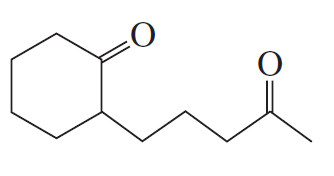
Problem 16a
How could each of the following compounds be prepared from cyclohexanone?
a.
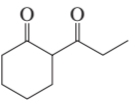
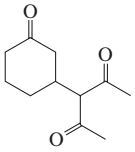
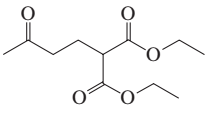
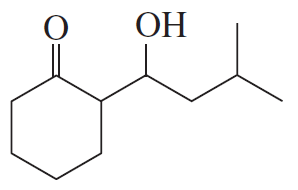
Problem 16b
How could each of the following compounds be prepared from cyclohexanone?
b.
Problem 17a
Describe how the following compounds could be prepared from cyclohexanone using an enamine intermediate:
a.
Problem 17b
Describe how the following compounds could be prepared from cyclohexanone using an enamine intermediate:
b.
Problem 18a
Draw the products of the following reactions:
a.
Problem 18b
Draw the products of the following reactions:
b.
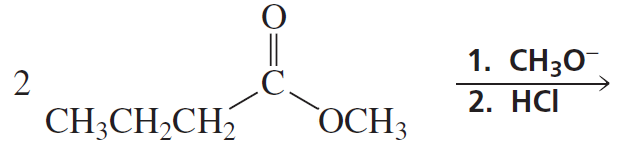
Problem 19a
What reagents should be used to prepare the following compounds?
a.
Problem 19b
What reagents should be used to prepare the following compounds?
b.
Problem 19c
What reagents should be used to prepare the following compounds?
c.
Problem 20a
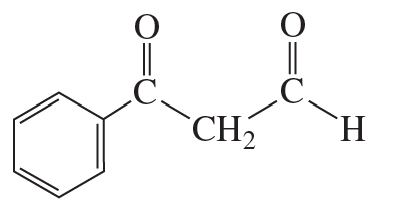
What aldol addition product is formed from each of the following compounds?
a.
Problem 20b
What aldol addition product is formed from each of the following compounds?
b.
Problem 24
How could you prepare the following compound using a starting material that contains no more than three carbons?
Problem 25a
Describe how the following compounds can be prepared using an aldol addition in the first step of the synthesis:
a.
Problem 25b
Describe how the following compounds can be prepared using an aldol addition in the first step of the synthesis:
b.
Problem 25c
Describe how the following compounds can be prepared using an aldol addition in the first step of the synthesis:
c.
Problem 26
What two carbonyl compounds are required for the synthesis of morachalcone A, via a Claisen–Schmidt condensation?
<IMAGE>
Problem 28a
Draw the products of the following reactions:
a.
Problem 28b
Draw the products of the following reactions:
b.
Problem 29a
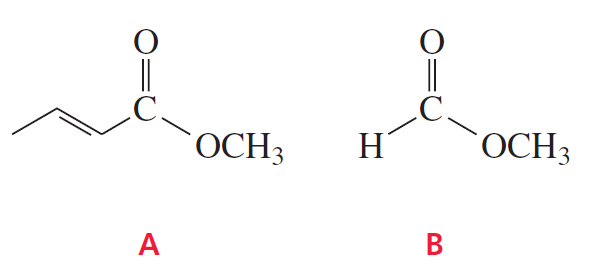
Which of the following esters cannot undergo a Claisen condensation?
Problem 31c
Show how each of the following compounds can be prepared from methyl phenyl ketone:
c.
Problem 32
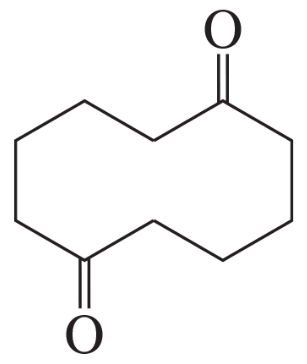
Write the mechanism for the reaction of a 1,7-diester with an alkoxide ion to form a cyclic b-keto ester.
Problem 34
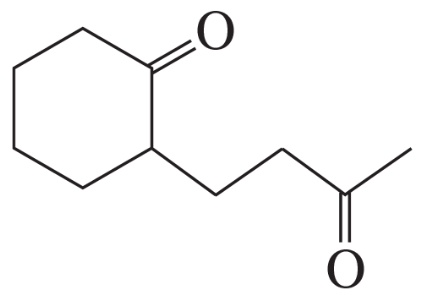
Can 2,4-pentanedione undergo an intramolecular aldol addition? If so, why? If not, why not?
Problem 35a
Draw the product of the reaction of each of the following compounds with a base:
a.
Problem 35b
Draw the product of the reaction of each of the following compounds with a base:
b.
Problem 35c
Draw the product of the reaction of each of the following compounds with a base:
c.
Problem 35d
Draw the product of the reaction of each of the following compounds with a base:
d.
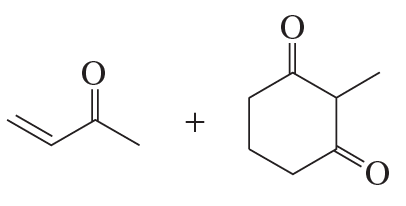
Problem 36a
Draw the product obtained by heating each pair of ketones in a basic solution.
a.