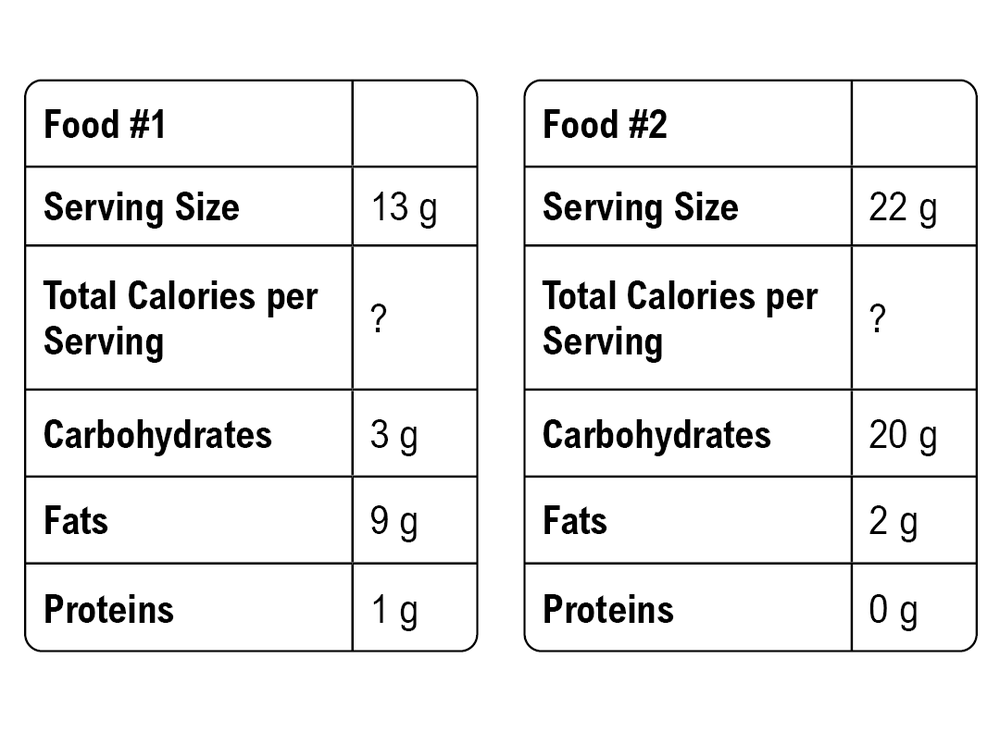
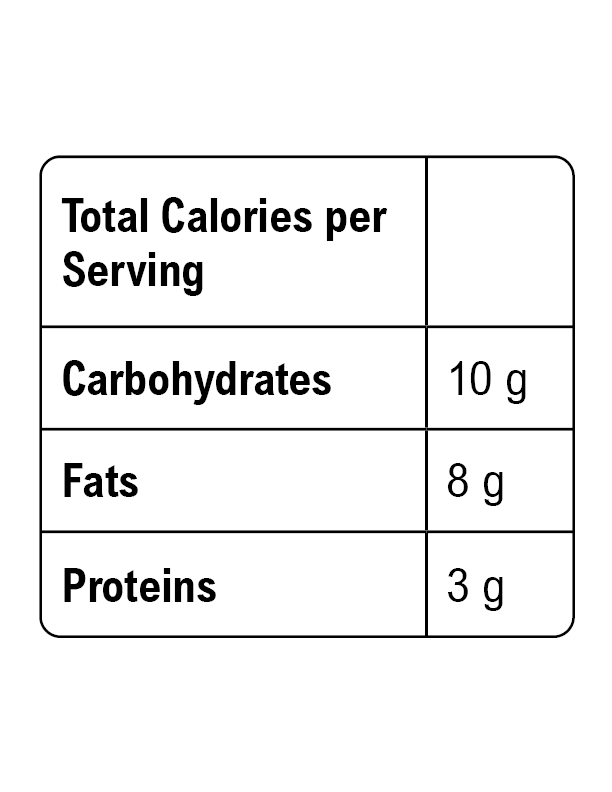
Energy in our food primarily comes from macronutrients, and we measure this energy in calories. Understanding the term metabolism is essential, as it refers to the set of chemical reactions that break down and utilize food molecules to release energy. The energy released through metabolism is quantified in calories.
There are two definitions of the term calorie: the lowercase 'c' calorie and the uppercase 'C' calorie. The lowercase 'c' calorie is defined as the energy required to heat one gram (or one milliliter) of water by one degree Celsius. This definition is commonly used in chemistry but is not typically applied in nutrition due to its relatively small energy value.
In nutrition, we use the uppercase 'C' calorie, which is equivalent to a kilocalorie (kcal). One kilocalorie equals 1,000 lowercase 'c' calories. For example, if one lowercase 'c' calorie can raise the temperature of one gram of water by one degree Celsius, then one kilocalorie can raise the temperature of one liter of water by the same amount. Nutrition labels refer to this uppercase 'C' calorie, even if they display it in lowercase.
To illustrate the energy content in food, consider a typical 2,000-calorie diet, which is often used as a benchmark. This amount of energy is sufficient to raise the temperature of ten two-liter bottles of water from freezing to boiling. In other words, if you were to combust the food consumed in a day, the energy released would be enough to boil 20 liters of water starting from freezing. This highlights the significant energy contained in our food, justifying the use of kilocalories in nutritional contexts.
As we progress, we will explore how to calculate the caloric content of food based on its macronutrient composition, enhancing our understanding of nutrition and energy balance.