Textbook Question
Draw the condensed structural or line-angle formulas for the products of the following:
c.

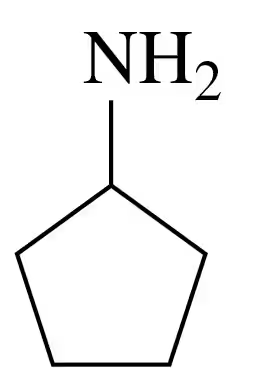
 Verified step by step guidance
Verified step by step guidance
Draw the condensed structural or line-angle formulas for the products of the following:
c.
Draw the condensed structural or line-angle formulas for the products of the following:
a.
Draw the condensed structural or line-angle formulas for the products of the following:
b.
Draw the condensed structural or line-angle formula if cyclic, for each of the following:
b. cyclohexylamine
Draw the condensed structural or line-angle formula if cyclic, for each of the following:
d. N-propylaniline
Draw the condensed structural or line-angle formula if cyclic, for each of the following:
d. ethylmethylammonium bromide