Textbook Question
What is the ester responsible for the flavor and odor of the following fruit?
c. apricot

 Verified step by step guidance
Verified step by step guidance
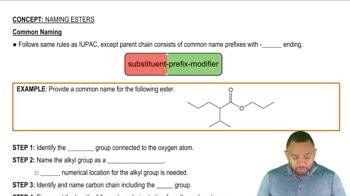
What is the ester responsible for the flavor and odor of the following fruit?
c. apricot
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
b. butyl formate
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
d. methyl propanoate
Draw the condensed structural formulas for a and b and line-angle formulas for c and d:
c. propyl benzoate
What are the products of the base hydrolysis of an ester?
Draw the condensed structural or line-angle formulas for the products from the acid- or base-catalyzed hydrolysis of each of the following:
b.