Textbook Question
Write the IUPAC name for each of the following: (12.3)
c. <IMAGE>

 Verified step by step guidance
Verified step by step guidance
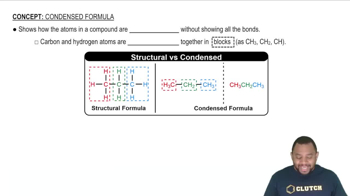
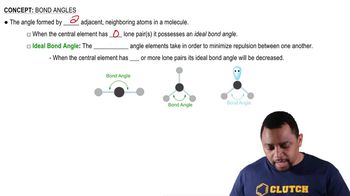
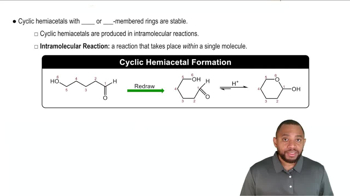
Write the IUPAC name for each of the following: (12.3)
c. <IMAGE>
Draw the condensed structural or line-angle formula, if cyclic, for each of the following:
a. 4-chlorobenzaldehyde
Draw the condensed structural or line-angle formula, if cyclic, for each of the following:
b. 3-chloropropionaldehyde
Draw the condensed structural or line-angle formula, if cyclic, for each of the following:
d. 3,5-dimethylhexanal
Draw the condensed structural or line-angle formula for the ketone or carboxylic acid product when each of the following is oxidized:
d.
Draw the condensed structural formulas and give the IUPAC names for all the alcohols that have the formula C5H12O.