Textbook Question
Give the IUPAC name for each of the following:
b.

 Verified step by step guidance
Verified step by step guidance
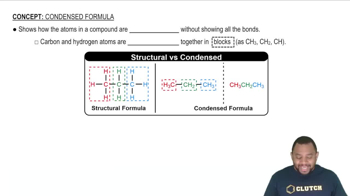
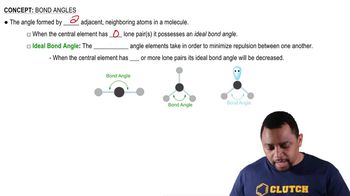
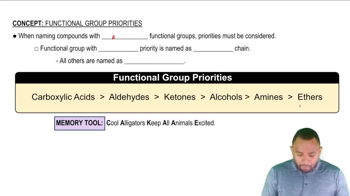
Give the IUPAC name for each of the following:
b.
Give the IUPAC name for each of the following:
d.
Draw the condensed structural formula, or line-angle formula if cyclic, for each of the following:
b. 3-pentanethiol
Draw the condensed structural formula, or line-angle formula if cyclic, for each of the following:
c. 2-methyl-2-butanol
Draw the condensed structural formula, or line-angle formula if cyclic, for each of the following:
d. 4-bromophenol
Draw the condensed structural formula, or line-angle formula if cyclic, for each of the following:
a. ethyl alcohol