Textbook Question
What are the products of the base hydrolysis of an ester?

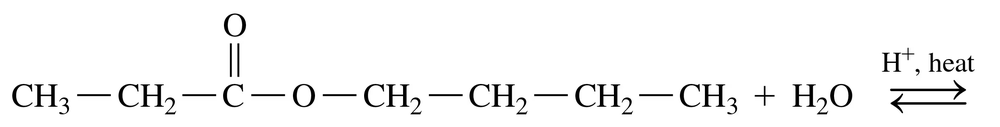
 Verified step by step guidance
Verified step by step guidance
What are the products of the base hydrolysis of an ester?
Draw the condensed structural or line-angle formulas for the products from the acid- or base-catalyzed hydrolysis of each of the following:
a.
Draw the condensed structural or line-angle formulas for the products from the acid- or base-catalyzed hydrolysis of each of the following:
b.
Draw the condensed structural or line-angle formulas for the products from the acid- or base-catalyzed hydrolysis of each of the following:
d.
Write the common name for each of the following:
b.
Write the common name for each of the following:
c.