Back
BackProblem 2b
Identify the type of particle or radiation for each of the following:
b. 11H
Problem 5c
Identify each of the following:
c. 10X
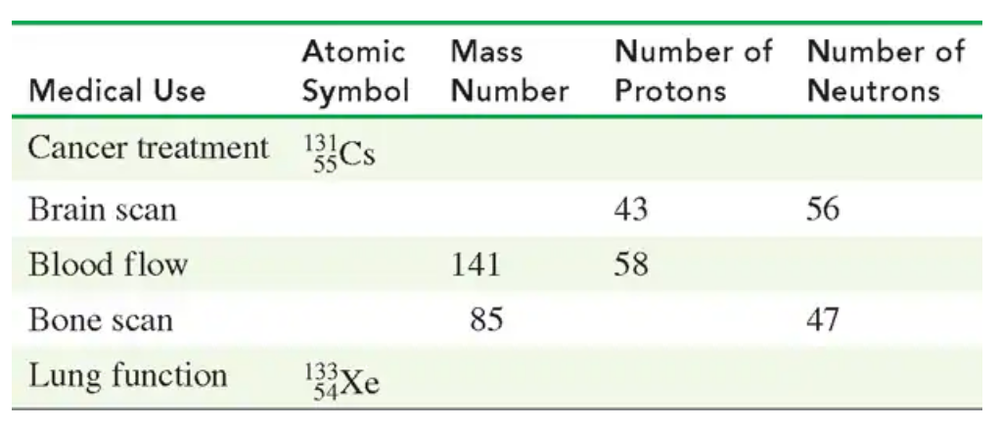
Problem 10
Supply the missing information in the following table:
Problem 11b
Match the type of radiation (1 to 3) with each of the following statements:
1. alpha particle
2. beta particle
3. gamma radiation
b. shielding protection includes lead or thick concrete
Problem 11c
Match the type of radiation (1 to 3) with each of the following statements:
1. alpha particle
2. beta particle
3. gamma radiation
c. can be very harmful if ingested
Problem 12c
Match the type of radiation (1 to 3) with each of the following statements:
1. alpha particle
2. beta particle
3. gamma radiation
c. travels only a short distance in air
Problem 19c
Complete each of the following nuclear equations and describe the type of radiation:
c. 6629Cu → 6630Zn + ?
Problem 19d
Complete each of the following nuclear equations and describe the type of radiation:
d. ? → 23490Th + 42He
Problem 19e
Complete each of the following nuclear equations and describe the type of radiation:
e. 18880Hg → ? + 0+1e
Problem 20e
Complete each of the following nuclear equations and describe the type of radiation:
e. ? → 8939Y + 0+1e
Problem 22d
Complete each of the following bombardment reactions:
d. ? + 6428Ni → 272111Rg + 10n
Problem 23a
Match each property (1 to 3) with its unit of measurement.
1. activity
2. absorbed dose
3. biological damage
a. rad
Problem 25
Two technicians in a nuclear laboratory were accidentally exposed to radiation. If one was exposed to 8 mGy and the other to 5 rad, which technician received more radiation?
Problem 26
Two samples of a radioisotope were spilled in a nuclear laboratory. The activity of one sample was 8 kBq and the other 15 mCi. Which sample produced the higher amount of radiation?
Problem 28a
The dosage of technetium-99m for a lung scan is 20. µCi/kg of body mass. How many millicuries of technetium-99m should be given to a 50.0-kg person (1 mCi = 1000 µCi)
Problem 28b
Suppose a person absorbed 50 mrad of alpha radiation. What would be the equivalent dose in millisieverts?
Problem 30c
For each of the following, indicate if the number of half-lives elapsed is:
1. one half-life
2. two half-lives
3. three half-lives
c. a sample of Au-198 with a half-life of 2.7 days after 5.4 days
Problem 33b
Strontium-85, used for bone scans, has a half-life of 65 days.
b. How long will it take for the radiation level of strontium-85 to drop to one-eighth of its original level?
Problem 34b
Fluorine-18, which has a half-life of 110 min, is used in PET scans.
b. If 100. mg of fluorine-18 is shipped at 8:00 a.m., how many milligrams of the radioisotope are still active when the sample arrives at the radiology laboratory at 1:30 p.m.?
Problem 35a
Bone and bony structures contain calcium and phosphorus.
a. Why would the radioisotopes calcium-47 and phosphorus-32 be used in the diagnosis and treatment of bone diseases?
Problem 35b
Bone and bony structures contain calcium and phosphorus.
b. During nuclear tests, scientists were concerned that strontium-85, a radioactive product, would be harmful to the growth of bone in children. Explain.
Problem 36a
Technetium-99m emits only gamma radiation. Why would this type of radiation be used in diagnostic imaging rather than an isotope that also emits beta or alpha radiation?
Problem 42
How does a chain reaction occur in nuclear fission?
Problem 44
In another fission reaction, uranium-235 bombarded with a neutron produces strontium-94, another small nucleus, and three neutrons. Write the balanced nuclear equation for the fission reaction.
Problem 45b
Indicate whether each of the following is characteristic of the fission or fusion process, or both:
b. The nuclear process occurs in the Sun.
Problem 51
In problems 5.51 to 5.54, a nucleus is shown with protons and neutrons.
Draw the new nucleus when this isotope emits a positron to complete the following:
<IMAGE>
Problem 52
In problems 5.51 to 5.54, a nucleus is shown with protons and neutrons.
Draw the nucleus that emits a beta particle to complete the following:
<IMAGE>
Problem 53
In problems 5.51 to 5.54, a nucleus is shown with protons and neutrons.
Draw the nucleus of the isotope that is bombarded in the following:
<IMAGE>
Problem 54
In problems 5.51 to 5.54, a nucleus is shown with protons and neutrons.
Complete the following bombardment reaction by drawing the nucleus of the new isotope that is produced in the following:
<IMAGE>
Problem 56b
Use the following decay curve for iodine-131 to answer problems a to c:
<IMAGE>
b. Complete the number of days on the horizontal axis.