Textbook Question
Identify each of the following as D or L:
a.

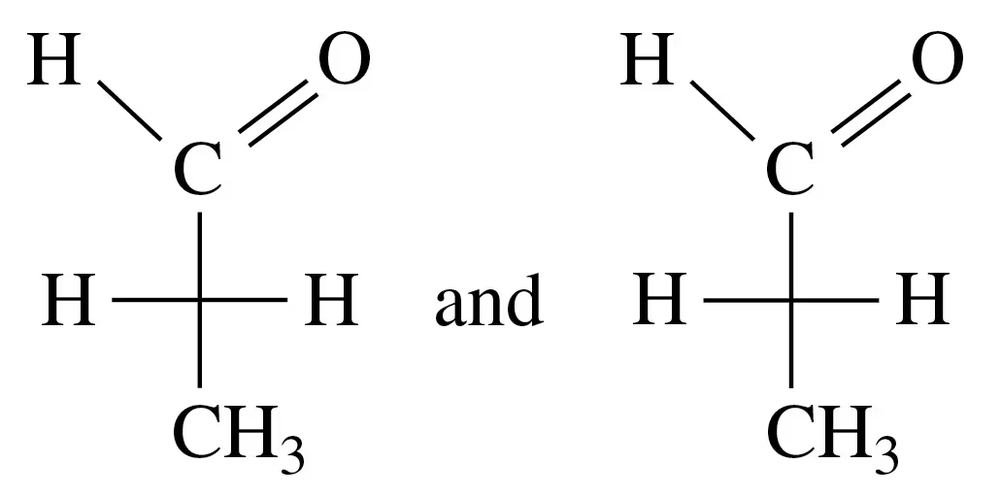
 Verified step by step guidance
Verified step by step guidance

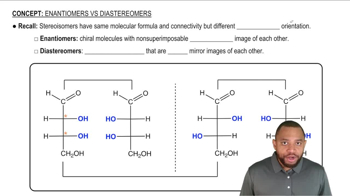
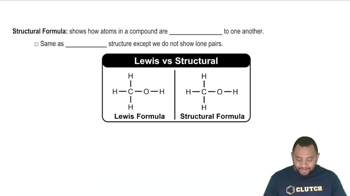
Identify each of the following as D or L:
a.
Draw the Fischer projection for each of the following wedge–dash structures:
a.
Draw the Fischer projection for each of the following wedge–dash structures:
a.
Indicate whether each pair of Fischer projections represents enantiomers or identical structures.
a.
Identify each of the following as the D or L enantiomer:
a.
Draw the Fischer projection for the other enantiomer of a to b in problem 13.21.
a.
b.