Give the name of one or more polysaccharides that matches each of the following descriptions:
d. produces maltose during digestion

 Verified step by step guidance
Verified step by step guidance

Give the name of one or more polysaccharides that matches each of the following descriptions:
d. produces maltose during digestion
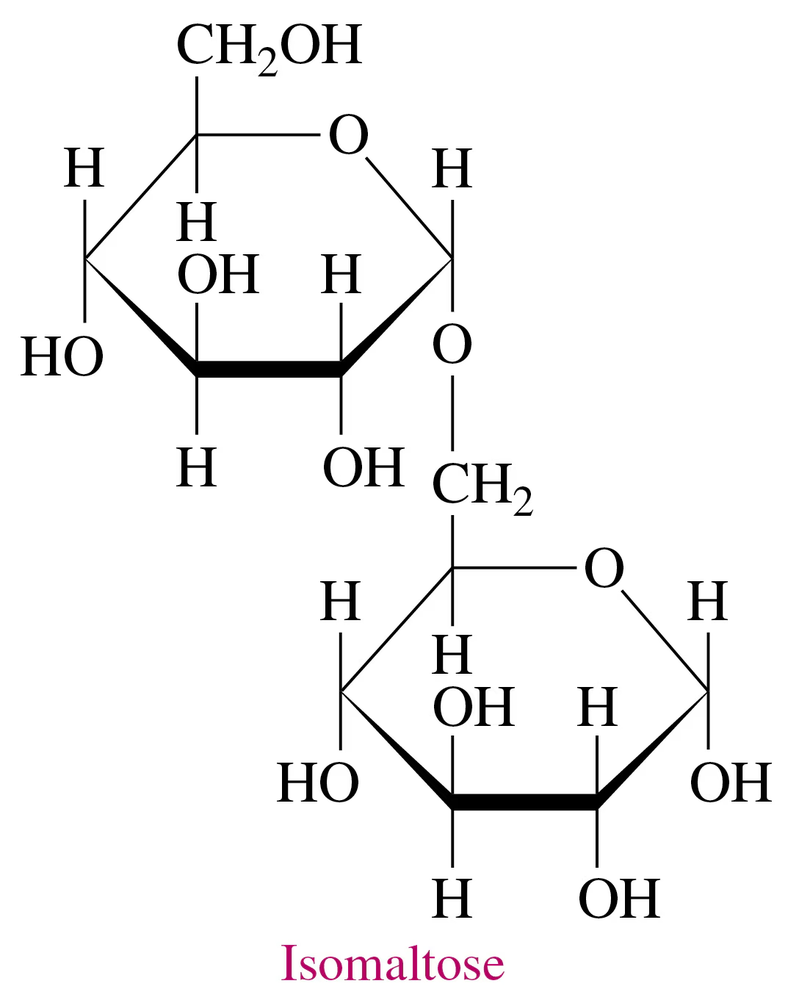
Isomaltose, obtained from the breakdown of starch, has the following Haworth structure:
a. Is isomaltose a mono-, di-, or polysaccharide?
Isomaltose, obtained from the breakdown of starch, has the following Haworth structure:
b. What are the monosaccharides in isomaltose?
Melezitose, a carbohydrate secreted by insects, has the following Haworth structure:
a. Is melezitose a mono-, di-, or trisaccharide?
Melezitose, a carbohydrate secreted by insects, has the following Haworth structure:
b. What monosaccharides are present in melezitose?
Melezitose, a carbohydrate secreted by insects, has the following Haworth structure:
c. Is melezitose a reducing sugar?