Which compound in each pair would be more soluble in water? Explain.
b. 2-propanol or 2-pentanol

 Timberlake 13th Edition
Timberlake 13th Edition Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones
Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones Problem 52a
Problem 52a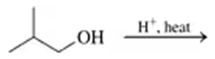
 Verified step by step guidance
Verified step by step guidance
Which compound in each pair would be more soluble in water? Explain.
b. 2-propanol or 2-pentanol
Which compound in each pair would be more soluble in water? Explain.
c. methyl propyl ether or 1-butanol
Draw the condensed structural or line-angle formula for the alkene, aldehyde, or ketone product of each of the following reactions:
a.
Draw the condensed structural or line-angle formula for the alkene, aldehyde, or ketone product of each of the following reactions:
c.
Draw the condensed structural or line-angle formula for the alcohol produced when hydrogen and a nickel catalyst reduce each of the following:
b.
Draw the condensed structural or line-angle formula for the alcohol produced when hydrogen and a nickel catalyst reduce each of the following:
a.