Back
BackProblem 35b
The following names are incorrect. What is wrong with each?
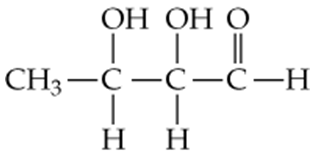
b. 2-Butanal
Problem 38
Which of the following compounds will react with Tollens' reagent? With Benedict's reagent?
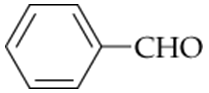
a. Cyclopentanon
b. Hexanal
c.
Problem 39a
Draw the structures of the products formed when the following compounds react with a reducing agent.
a.
Problem 43a
Write the structures of the hemiacetal or hemiketal that result from reactions (a) and (b). Write the structures of the complete hydrolysis products of the acetal or ketal in (c) and (d).
a. Acetone + Ethanol → ?
Problem 44
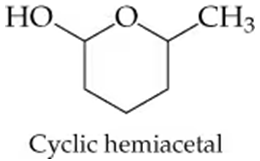
Cyclic hemiacetals commonly form if a molecule has both an alcohol group and a carbonyl group elsewhere in the same molecule, especially if they are four or five carbons apart. What is the structure of the hydroxy aldehyde from which this hemiacetal might form?
Problem 46
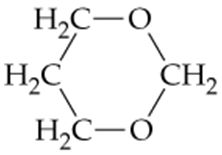
What two products result from the complete hydrolysis of this cyclic acetal?
Problem 47b
Acetals and ketals are usually made by reaction of an aldehyde or ketone with two molecules of a monoalcohol. If an aldehyde or ketone reacts with one molecule of a dialcohol, however, a cyclic acetal or ketal results.
b. Draw the cyclic ketal formed when the hemiketal from part (a) reacts with the ―OH labeled in blue.
Problem 48
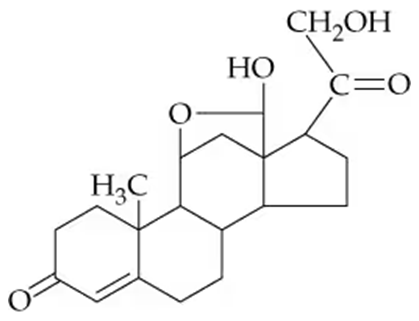
Aldosterone is a key steroid involved in controlling the sodium–potassium balance in the body. Identify the functional groups in aldosterone.
Problem 49
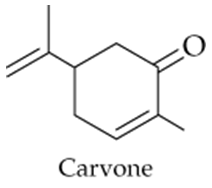
The compound carvone is responsible for the odor of spearmint. Identify the functional groups in carvone.
Problem 51
Can the alcohol (CH3)3COH be formed by the reduction of an aldehyde or ketone? Why or why not?
Problem 52
Many flavorings and perfumes are partially based on fragrant ketones, with far fewer being based on fragrant aldehydes. Why do you think ketones are used more frequently than aldehydes? See Section 15.5 for a clue.
Problem 56c
Draw the structural formulas of the following compounds:
c. 2-Methoxy-2-methylpropane
Problem 61
The liquids 1-butanol and butanal have similar molar masses. Which is expected to have the higher boiling point? Explain your choices.
Problem 63
Draw all the ketones you can with a chemical formula of C8H16O whose longest chain is eight carbons. Name each using both its IUPAC and common name.
Problem 64
In Problem 15.24, you were given the structure of the free aldehyde form of glucose. Try to draw the two cyclic hemiacetal forms of glucose you would get if (a) the OH on C4 formed the ring and (b) the OH on C3 formed the ring.