Textbook Question
Two electrostatic potential maps are shown, one of methyl-lithium (CH3Li) and the other of chloromethane (CH3Cl). Based on their polarity patterns, which do you think is which? (a)
(b)


 Verified step by step guidance
Verified step by step guidance
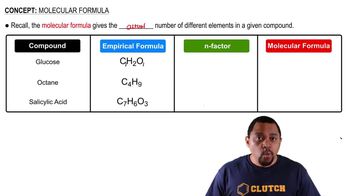
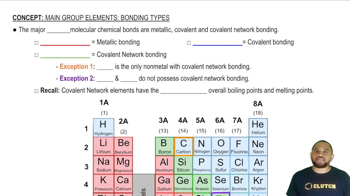
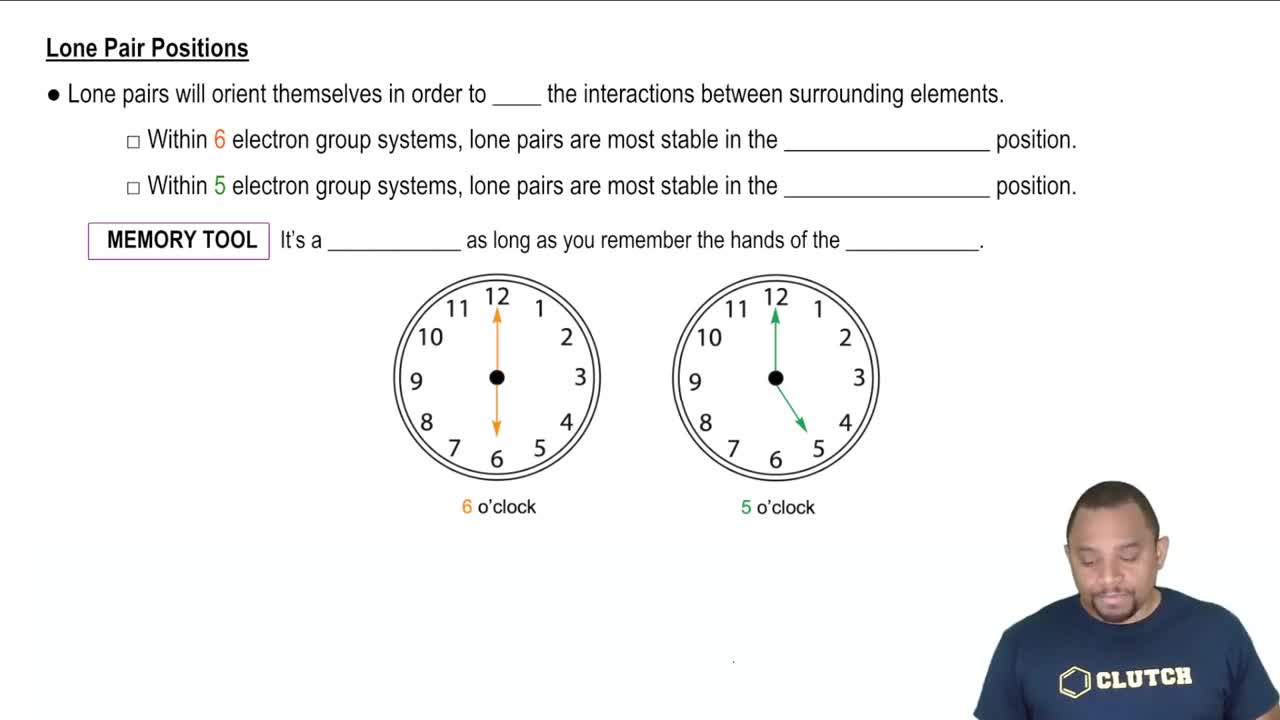
Two electrostatic potential maps are shown, one of methyl-lithium (CH3Li) and the other of chloromethane (CH3Cl). Based on their polarity patterns, which do you think is which? (a)
(b)
Electrostatic potential maps of acetaldehyde (C2H4), ethane (C2H6), ethanol (C2H6O), and fluorethane (C2H5F) are shown. Which do you think is which? (a)
(b)
(c)
(d)